Category: Toxicology
Keywords: laboratory, lipid, toxicology (PubMed Search)
Posted: 12/10/2015 by Bryan Hayes, PharmD
Click here to contact Bryan Hayes, PharmD
The American Academy of Clinical Toxicology's Lipid Emulsion workgroup has published its first of 4 systematic reviews on the use of lipid emulsion in toxicology, this one on lipid's effect on laboratory analyses. [1] As expected, administering a fat bolus can significantly alter labs drawn subsequently.
The key point: If you are considering lipid for overdose, draw labs prior to giving it.
Which labs are affected? Most. Here's a helpful mnemonic courtesy of Dr. Kyle DeWitt.
Also remember to give lipid in its own line. It isn't compatable with most resuscitation drugs. [2]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: CO, Carbon Monoxide Detector (PubMed Search)
Posted: 12/3/2015 by Kathy Prybys, MD
Click here to contact Kathy Prybys, MD
Carbon monoxide (CO) is a colorless, odorless, tasteless toxic gas produced by incomplete combustion in fuel-burning devices and is a leading cause of poisoning morbidity and mortality.
Symptoms can be easily misinterpreted (e.g., headache, nausea, dizziness, or confusion) thus victims may not realize they are being poisoned.
CO detectors use an audible alarm and are effective in alerting potential victims of presence of CO. Some versions offer a digital readout of the CO concentration. Detectors are not a simple alarm level (as in smoke detectors) but are a concentration-time function.
In the UL 2034 Standard, Underwriters Laboratories specifies response times for CO alarms:
Current Occupational Safety and Health Administration permissible exposure limit for CO is 50 parts per million as an 8-hour time-weighted average concentration.
CO detectors have a limited lifespan of up to 7 years.
Forty percent of residential detectors studied failed to alarm in hazardous concentrations, despite outward indications that they were operating as intended.
CO detectors 10 years and older had the highest failure rates.
Night of sirens: analysis of carbon monoxide-detector experience in suburban Chicago. Bizovi KE, Leikin JB, Hryhorczuk DO, Frateschi LJ. Ann Emerg Med. 1998;31(6):737 740.
Residential carbon monoxide detector failure rates in the United States. Ryan TJ, Arnold KJ. Am J Public Health. 2011 Oct;101(10):e15-7. doi:10.2105/AJPH.2011.300274. Epub 2011 Aug 18.
Deaths from unintentional carbon monoxide poisoningand potential for prevention with carbon monoxide detectors. Yoon SS, Macdonald SC, Parrish RG. JAMA. 1998 Mar 4;279(9):685-7.
Category: Toxicology
Keywords: e-cigarettes, liquid nicotine, nicotine toxicity (PubMed Search)
Posted: 11/19/2015 by Hong Kim, MD
Click here to contact Hong Kim, MD
Electronic cigarettes have been gaining popularity in the U.S. as a smokeless delivery system for nicotine. These devices require liquid nicotine (e-liquid) that are vaporized and inhaled (vaping).
E-liquid can have nicotine concentration as high as 100 mg/mL, which are diluted prior to use. When ingested in high concentration and in sufficient volume (1 vial = 15 mL) patients can develop significant nicotinic toxicity. Recently a case of cardiac arrest has been reported after ingesting two 15 ml vial (100 mg/mL).
Nicotine mimics the effects of acetylcholine (Ach) release by binding to nicotinic receptors located in:
Clinical manifestation of toxicity (similar to cholinergic toxidrome) is biphasic with early central stimulation followed by depression. (see table below)
|
| GI | Respiratory | Cardiovascular | Neurologic |
| Early (1 hr) | Nausea Vomiting Salivation Abdominal pain | Bronchorrhea Hyperpnea | Hypertension Tachycardia Pallor | Agitation Anxiety Dizziness Blurred vision Headache Hyperactivity Tremors Fasciculation Seizures |
| Late (0.5-4 hr) | Diarrhea | Hypoventilation Apnea | Bradycardia Hypotension Dysrhythmias Shock | Lethargy Weakness Paralysis |
Management: There is no specific antidote or reversal agent. The management of nicotine toxicity focuses on organ-specific dysfunction.
Category: Toxicology
Keywords: Andexanet, apixaban, rivaroxaban, factor Xa (PubMed Search)
Posted: 11/12/2015 by Bryan Hayes, PharmD
Click here to contact Bryan Hayes, PharmD
Not to be outdone by the recent FDA approval of Idarucizumab to reverse dabigatran, a new factor Xa reversal agent is under investigation. "Andexanet binds and sequesters factor Xa inhibitors within the vascular space, thereby restoring the activity of endogenous factor Xa and reducing levels of anticoagulant activity, as assessed by measurement of thrombin generation and anti factor Xa activity, the latter of which is a direct measure of the anticoagulant activity."
Design
Two parallel randomized, placebo-controlled trials (ANNEXA-A [apixaban] and ANNEXA-R [rivaroxaban]) were conducted in healthy vounteers to evaluate the ability of andexanet to reverse anticoagulation, as measured by the percent change in anti factor Xa activity after administration.
What they Found
Compared to placebo, andexanet significantly reduced anti-factor Xa activity, increased thrombin generation, and decreased unbound drug concentration in both the apixaban and rivaroxaban groups.
Application to Clinical Practice
Siegal DM, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. November 11, 2015.
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: THC, Spice, JWH (PubMed Search)
Posted: 11/5/2015 by Kathy Prybys, MD
(Updated: 11/6/2015)
Click here to contact Kathy Prybys, MD
Designer drugs are structural or functional analogs of controlled substances produced to mimic pharmacological effects of the original compound while circumventing legal restrictions and detection on drug screens. Considered "legal highs" by the public, these highly potent drugs are produced in clandestine laboratories with no regulations for quality control or clinical testing for phamacological effects and thus present major threat to public health. Examples include synthetic hallucinogens (DOM: STP), opiates ( methylfentanyl:china white), stimulants (methamphetamine:crank, MDMA: ecstasy, cathinones:bath salts) and synthetic cannabinoids (spice).
The synthetic cannabinoids are the newest designer drugs and numerous cases of intoxication are being reported including some fatalties.Cannabinoids fall into 3 classes: endocannabinoids, phytocannabinoids, synthetic. Marijuana, the best known cannabinoid is plant derived and its psychoactive effects are mainly due to delta-9-tetrahydrocannabinol (THC) which binds with the endocannabinoid receptors CB1 and CB2 found throughout the central and peripheral nervous system and peripheral organs. The CB receptors interact with opiate receptors which is likely responsible for the analgesic effect.
Since 1984, the John Huffman research group at Clemenson University synthesized over 450 cannabinoid compounds for biomedical reseach known as "JWH compounds". These compounds hold great promise in the investigation of multiple diseases and development of new novel therapies. Over the last several years, these cannabinoid compounds began cropping up sprayed onto herbs marketed in colorful packets and sold on the internet, convienence stores, and head shops. Although clearly labeled as "not for human consumption" considered on the street as a legal alternative to marijuana.
Key Points:
Seely KA, Lapoint , et al. Spice drugs are more than harmless herbal blends: a review of pharmacology and toxicology of synthetic cannabinoids. Progress in Neuropharmacology & Biological Psychiatry (2012), doi:10.1016/j.pnpbp.2012.04.017
Wiley JL. Marusich JA. et al. Hijacking of basic research: the case of synthetic cannabinoids. Methods Rep RTI Press. 2011 November; 2011; .doi: 10.3768/rtipress.2011.op.0007.1111
Category: Toxicology
Keywords: propranolol, lipid emulsion (PubMed Search)
Posted: 10/22/2015 by Fermin Barrueto
Click here to contact Fermin Barrueto
There have been a variety of case reports that have been describing the effects of lipid emulsion therapy on severe hemodynamic overdoses. As time has gone on, we have realized that this therapy is not for all severe overdoses. The type of medication and its pharmacokinetic properties factor into the decision. There is minimal evidence and no ideal randomized control trials that will tell us what the right answer is but take beta-blockers for instance:
Atenolol - in overdose, consider hemodialysis, very effectively removed by HD [1]
Propranolol - very lipophilic and one of the few beta-blockers that can cause widened QRS, seizures as well as the prototypical hypotension and bradycardia.
Because of its lipophilicity, ability to cross the blood brain barrier and ability to cause lethal dysrrthmias, lipid emulsion therapy has been effective in reversing the clinically severe effects of a propranolol overdose. [2]
1)Case report: atenolol overdose successfully treated with hemodialysis.
Huang SH, Tirona RG, Ross C, Suri RS.
Hemodial Int. 2013 Oct;17(4):652-5. doi: 10.1111/hdi.12020. Epub 2013 Jan 24.
Jovic-Stosic J, Gligic B, Putic V, Brajkovic G, Spasic R.
Clin Toxicol (Phila). 2011 Jun;49(5):426-30. doi: 10.3109/15563650.2011.583251.
Category: Toxicology
Keywords: body stuffer, body packer, CAT Scan (PubMed Search)
Posted: 10/15/2015 by Hong Kim, MD
Click here to contact Hong Kim, MD
Toxicity due to body packing and body stuffing can be a significant concern due to unknown quantity and/or substance that was ingested.
A recent prospective observational case series compared the utility of CT abdomen/pelvis with and without PO contrast in identifying the ingested packets.
The gold standard comparison: surgical removal or expulsion of packets.
All patients received CT abd/pelvis with and without PO contrast.
A. Body stuffers (n = 24)
CT w/ PO contrast:
Positive: 7 (sensitivity 29.2%)
Negative: 17
CT w/o PO contrast:
Positive: 9 (sensitivity 36.5%)
Negative: 15
All 24 patients passed ingested packets
B. Body packers (n= 11)
CT w/ PO contrast
CT w/p PO contrast
10 patients expulsed packets; one patient did not have any packets.
Conclusion
Bottom line:
Shahnazi M et al. Comparison of abdominal computed tomography with and without oral contrast in diagnosis of body packers and body stuffers. Clin Toxicol 2015;53:596-603.
Category: Toxicology
Keywords: hemodialysis, dabigatran, rebound (PubMed Search)
Posted: 10/7/2015 by Bryan Hayes, PharmD
(Updated: 10/8/2015)
Click here to contact Bryan Hayes, PharmD
In patients receiving renal replacement therapy as a treatment modality for dabigatran-related bleeding, watch for a rebound concentration increase after hemodialysis is stopped.
More than 50% of patients demonstrate a rebound effect with a median increase in dabigatran concentration of 33%.
It is unclear whether this rebound effect is clinically important, and whether it translates to prolonged clinically relevant bleeding. Extended hemodialysis sessions or consideration of CVVHD should offset this potential problem.
Bonus Pearl:
The North American Congress of Clinical Toxicology starts today and runs through October 12. Look for toxicology pearls and updates on Twitter under the official conference hashtag #NACCT15.
Chai- Adisaksopha C, et al. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost 2015;13(10):1790-8. [PMID 26270886]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: Odansetron, Zofran (PubMed Search)
Posted: 10/2/2015 by Kathy Prybys, MD
Click here to contact Kathy Prybys, MD
Category: Toxicology
Keywords: flushed skin (PubMed Search)
Posted: 9/16/2015 by Hong Kim, MD
(Updated: 2/9/2026)
Click here to contact Hong Kim, MD
Monosodium glutamate
Metabisulfites (Na sulfite, Na/K bisfulfite, Na/K metabisulfite, etc.)
Tyramine reaction
Niacin
Trichloroethylene
Scrombroids
Hydroxocobalamin
Category: Toxicology
Keywords: eye drops, pupil size, ophthalmic (PubMed Search)
Posted: 9/8/2015 by Bryan Hayes, PharmD
(Updated: 9/11/2015)
Click here to contact Bryan Hayes, PharmD
In the evaluation of ED patients, it may be important to understand the effect on pupil size from the ophthalmic medications they use. Here is a summary chart of common eye drops and their effect on pupil size.
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: body stuffers, observation period (PubMed Search)
Posted: 8/20/2015 by Hong Kim, MD
(Updated: 2/9/2026)
Click here to contact Hong Kim, MD
People who hide illicit drugs can be classified in to three different types.
1. Body stuffers – people who ingest drugs that are poorly wrapped to “eliminate” evidence from police – e.g. street dealers.
2. Body packers – people who ingest large amounts of “well” packed drug packets to transport drugs (usually internationally) – aka “mule.”
3. Body pushers – people hiding drugs in rectum or vagina.
Body stuffers are more frequently encountered in local ED compared to body packers. Stuffers can become symptomatic as the ingested drugs (cocaine, heroin, amphetamines) are often poorly wrapped (e.g. in plastic bag/wrap, cellophane paper, aluminium oil, etc.).
Recent retrospective article looked at the utility of 6-hour observation period in the ED as a management strategy for body stuffers. (n=126)
Characteristics
1. Ingested drugs (self-reported): heroin (48%), cocaine (46%), other drugs [cannabis, MDMA, diazepam, methamphetamine] (16%), unknown (8%)
2. Time of ingestion to ED presentation
Clinical findings
76% of the patients experience clinical signs of toxidrome at time of presentation.
Most common findings:
Patients who ingested heroin were more symptomatic vs. cocaine (87% vs. 70%)
Patients were discharged:
Conclusion
Yamamoto T et al. Management of body stuffers presenting to the emergency department. Europ J Emerg Med. May 8 [Epub ahead of print] PDIM: 25969343
Category: Toxicology
Keywords: flumazenil, benzodiazepine, overdose (PubMed Search)
Posted: 8/7/2015 by Bryan Hayes, PharmD
(Updated: 8/13/2015)
Click here to contact Bryan Hayes, PharmD
Flumazenil is generally avoided in most adult patients with suspected benzodiazepine overdose due to resedation, seizures/withdrawal, inconsistent reversal of respiratory depression, and the potential for proconvulsant coingestants.
Three relatively recent poison center studies have attempted to demonstrate the safety of flumazenil in this setting. [1-3] In the first study there were 904 adult patients with 13 reported seizures and 1 death. [1] A second study specific to pediatric patients reported 83 patients with no seizures and no deaths. [2] A third study found 80 patients with 1 seizure and 0 deaths. [3]
On the surface, it may appear that flumazenil is safe to give. But, retrospective poison center studies from voluntary reporting cannot be used to prove a drug's safety. The true denominator is unknown. In the pediatric study, we wouldn't expect children to experience withdrawal since they aren't on chronic benzodiazepine therapy. [2] So, it's no surprise there weren't any seizures or deaths.
A recent systematic review and meta-analysis of randomized trials summed it up perfectly: "Flumazenil should not be used routinely, and the harms and benefits should be considered carefully in every patient." [4] Cases in which to consider flumazenil are pediatric patients and reversal of procedural sedation if needed.
Follow me on Twitter (@PharmERToxGuy) and Google Plus (+bryanhayes13)
Category: Toxicology
Posted: 8/6/2015 by Kathy Prybys, MD
Click here to contact Kathy Prybys, MD
Poison ivy, oak, and sumac (Toxicodendron sp) causes a highly puritic, allergic contact dermatitits (ACD) that affects between 10 and 50 million in the US every year. It is a significant occupational hazard as well a scourge for outdoor enthusiasts.
Toxicodendron species contain oleoresins, known as Urushiol compound, secreted by all parts of the plant. Contact with the oil usually occurs by brushing against or direct handling of the plant or contaminated items. This toxin triggers a type IV delayed hypersensitivity reaction in approximately 75% of the population. Within 12-24 hours an erythematous, often linear, vesicular rash develops but new lesions can occur up to 2 weeks later.
There is no ideal treatment for ACD induced by Toxicodendron species. Avoidance and barrier protection are the best strategies. Recommended medications include antihistamines, topical preparations, and systemic steroids. However, steroids require a 2-3 week course to prevent recrudescence of the rash and are not without undesirable side affects.
Zanfel, an OTC granular polyethlene paste, removes urushiol by binding with it to create an aggregate cluster that can be washed away with water. It is highly effective, providing rapid relief even as a sole agent but requires multiple initial applications and is expensive. Mean Green hand scrub has similar ingredients and is claimed to bond urushiol also. Excessive scrathing and abrasive scrubs can cause secondary cellulitis requiring antibiotics.
Category: Toxicology
Keywords: sulfonylurea, hypoglycemia, octreotide (PubMed Search)
Posted: 7/28/2015 by Hong Kim, MD
(Updated: 2/9/2026)
Click here to contact Hong Kim, MD
Oral hypoglycemic agents (e.g. sulfonylureas) can cause symptomatic hypoglycemia. Unlike metformin, sulfonylureas stimulate the release of insulin from beta-cells (in pancreas) in response to serum glucose level.
ED management of hypoglycemia involves:
However, for recurrent hypoglycemia (> 3 episodes of hypoglycemia), think about octreotide, rather than starting a dextrose (D5) infusion.
For example, D5 infusion at 150 mL/hour has only 7.5 gm of dextrose (calculation: D5% = 5gm/100 mL). One gram of dextrose contains about 4 calories (equivalent to one piece of Skittles) So, with a D5 infusion at 150 mL/hour, you are giving your patients 8 pieces of Skittles per hour. A bottle of Snapple lemon ice tea (non-diet) has more calories (150 calories in 16 oz. or 473 mL)!
Octreotide 50 mcg SQ (q6 hour) injection will decrease the insulin release from the beta-cell by blocking the voltage-gated Ca channel on the beta-cell.
All patient who received octreotide in the ED requires admission to the hospital for observation. Patients can be safely discharge from the hospital when finger stick glucose level remains normal for 24 hours after the last dose of octreotide.
Bottom line: In sulfonylrea-induced recurrent hypoglycemia, administer octreotide, rather than continuous infusion of dextrose (D5) solution.
Category: Toxicology
Keywords: physostigmine, anticholinergic toxicity, TCA overdose, asystole (PubMed Search)
Posted: 7/16/2015 by Hong Kim, MD
(Updated: 2/9/2026)
Click here to contact Hong Kim, MD
Physostigmine is a cholinergic agent (acetylcholine esterase inhibitor) that can be used to reverse anticholinergic toxicity. Its use has been declining since the publication of several case reports of physostigmine induced cardiac arrest in tricyclic antidepressant (TCA) overdose.
The first case report (and often cited) was by Pental P. et al. (Ann Emerg Med 1980), who presented 2 cases (32 and 25 year old) of asystole after administration of physostigmine (2 mg) in severe TCA overdose. These two cases both had widened QRS interval (120, 240 msec) due to TCA poisoning. Bradycardia preceded the asystole.
The second case report (Shannon M Pediatr Emerg Care 1998) reported a 15 year-old girl with QRS widening (120 msec) received 2 mg of physostigmine and developed severe bradycardia and then asystole.
Another case series (Knudson K et al. BMJ 1984) of 41 patients with overdose of maprotiline showed that physostigmine administration was associated with higher incidence of seizures. No asystole was noted.
Today physostigmine is contraindicated in TCA poisoning. But if we think about it, physostigmine administration probably wasn’t a good idea in the first place. Correcting anticholinergic toxicity of TCA has limited benefit; mortality from TCA overdose is usually associated with cardiac toxicity (Na-channel blockade) and should be treated with NaHCO3 administration
Physostigmine still has a role in treating isolated anticholinergic toxicity (e.g. diphenhydramine, benztropine, dimenhydrinate, scopolamine, jimson weed overdose). Prior to physostigmine administration:
Bottom line: If you suspect isolated anticholinergic toxicity, think about physostigmine. Like any medication, risk and benefit of administration should be considered prior to administration.
Category: Toxicology
Keywords: dabigatran, bleeding, idarucizumab, reversal (PubMed Search)
Posted: 7/6/2015 by Bryan Hayes, PharmD
(Updated: 7/9/2015)
Click here to contact Bryan Hayes, PharmD
The New England Journal of Medicine and Lancet both published studies evaluating idarucizumab for reversal of dabigatran. It is a monoclonal antibody fragment that binds dabigatran with high affinity. Dr. Ryan Radecki summarizes the two articles on his EM Lit of Note blog.
Here are a few take home points from these early studies:
Pollack CV, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015 Jun 22. [Epub ahead of print, PMID 26095746]
Glund S, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in health male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015 Jun 15. [Epub ahead of print, PMID 26088268]
Follow me on Twitter (@PharmERToxGuy) or Google Plus (+bryanhayes13)
Category: Toxicology
Keywords: Synthetic cannabinoid, K2 (PubMed Search)
Posted: 6/18/2015 by Hong Kim, MD
(Updated: 2/9/2026)
Click here to contact Hong Kim, MD
Recently, there has been a surge in synthetic cannabinoid in the U.S., including the Baltimore area. According to U.S. poison control center data, there has been 229% increase in calls related to SC between January to May of 2015 compared to similar time period in 2014.
The most commonly reported adverse/clinical effects included:
End-organ injuries have been also reported in case reports, including AKI, seizure, MI, and CVA.
Synthetic cannabinoid includes a list of chemical compounds that are structurally different compared to THC – the active compound in marijuana. However, they possess full CB1 (cannabinoid) receptor agonism effect, unlike the THC, which is a partial CB1 receptor agonist.
These chemicals (particularly JWH series) were originally synthesized to study the effect of cannabinoid receptors. Overall, it is difficult to identify the compound and the dose within each packets of SC.
Commonly marketed names include: Spice, K2, K9, herbal highs, Scooby snax, WTF.
Table. Identified synthetic cannabinoids
| Chemical name | Chemical origin |
| JWH-018; JWH-073; JWH-250 | Laboratory of J.W. Huffman |
| CP47,497; CP47,497-C8; CP59,540; cannabicyclohexanol | Pfizer laboratory |
| HU-210 | Hebrew University laboratory |
| Oleamide | Fatty acid |
| UR-144 | CB2 receptor agonist |
| XLR-11, AKB-48, AM-2201, AM-694 |
|
Management: Majority of the patients with acute SC intoxication mostly requires supportive care, including benzodiazepine for acute agitation. However, ED providers should be mindful of potential end-organ injury.
Law R et al. Increase in reported adverse health effects related to synthetic cannabinoid use - United States, January - May 2015. MMWR 2015;64:618-619.
Weaver et al Designer drugs 2015: assessment and management. Addic Sci Clin Pract. 2015 Mar 25;10:8. doi: 10.1186/s13722-015-0024-7.
Takematsu M et al. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin Toxicol (Phila) 2014;59:973-975.
Buser GL et al. Acute kidney injury associated with smoking synthetic cannabinoid. Clin Toxicol 2014;52:664-73.
Category: Toxicology
Keywords: aspirin, extracorporeal, salicylate, poisoning (PubMed Search)
Posted: 5/22/2015 by Bryan Hayes, PharmD
(Updated: 6/11/2015)
Click here to contact Bryan Hayes, PharmD
The Extracorporeal Treatments in Poisoning (EXTRIP) Workgroup has published their latest review, this time on extracorporeal treatment for salicylate poisoning. Here are their recommendations on when to dialyze:
Juurlink DN, et al. Extracorporeal Treatment for Salicylate Poisoning: Systematic Review and Recommendations From the EXTRIP Workgroup. Ann Emerg Med. 2015 May 8. [Epub ahead of print, PMID 25986310]
Follow me on Twitter (@PharmERToxGuy) or Google Plus (+bryanhayes13)
Category: Toxicology
Posted: 6/5/2015 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Electronic cigarettes are battery-powered devices that deliver nicotine, flavorings, (e.g. fruit, mint, and chocolate), and other chemicals via an inhaled aerosol. E-cigarettes are currently not regulated by the FDA. In many states, there are no restrictions on the sale of e-cigarettes to minors.
Electronic cigarette exposures involving young children are rapidly increasing. Such exposures tend to involve patients aged < 5 years and occur by ingestion of the nicotine-containing liquid. There is a potential for acute nicotine toxicity (nausea, vomiting, pallor, diaphoresis, tachycardia, hypertenstion initially). Respiratory muscle weakness with respiratory arrest is the most likely cause of death.
To date, the overwhelming majority of pediatric ingestions have not resulted in serious medical outcomes. The most commonly reported adverse events were nausea and vomiting.
However, in May of 2014, the first pediatric case of toxicity from ingestion of e-cigarette nicotine liquid was reported. A 10-month old ingested an unknown amount of e-liquid and developed vomiting, tachycardia, grunting respirations, and ataxia. The symptoms resolved by 6 hours after ingestion without specific treatment.
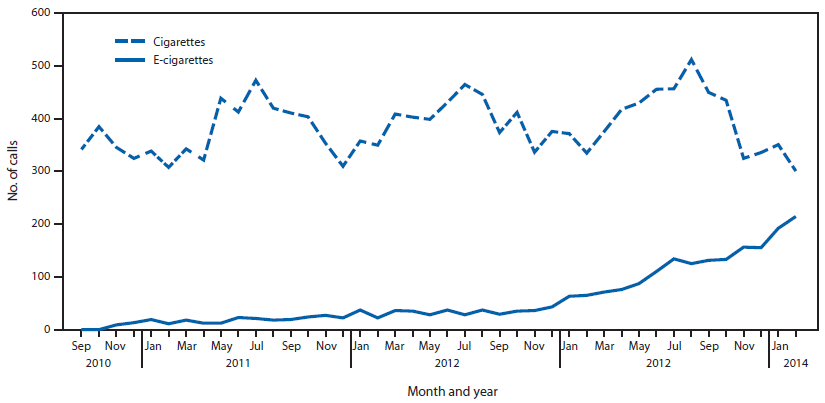
(1) The figure above shows the number of calls to poison centers for cigarette or e-cigarette exposures, by month, in the United States during September 2010 February 2014. E-cigarette exposure calls per month increased from one in September 2010 to 215 in February 2014.
(1) Chatham-Stephens K, Law R, Taylor E, et al. MMWR Morb Mortal Wkly Rep 2014;63:292-293.
(2) LoVecchio F, Zoph O. Incidence of electronic cigarette exposure in children skyrockets in Arizona. Am J Emerg Med, epub, 2/25/15.
(3) Bassett RA, Osterhoudt K, Brabazon T. Nicotine Poisoning in an Infant. N Engl J Med 2014;370:2249-2250.
