Category: Toxicology
Keywords: medetomadine, withdrawal (PubMed Search)
Posted: 2/4/2026 by Robert Flint, MD
(Updated: 2/5/2026)
Click here to contact Robert Flint, MD
The US drug supply has been found to contain medetomidine as an adulterant to heroine/fentanyl. It is a potent tranquilizer used in animals. It is an alpha 2 blocker (similar pharmacology to clonidine and xylazine). Exposure to this drug can induce withdrawal symptoms to include anxiety, tremor, diaphoresis, nausea, vomiting, agitation, sympathetic hyperactivity, and delirium. Withdrawal can start within 4-6 hours of last use.
Treatment for withdrawal is outlined in this diagram.
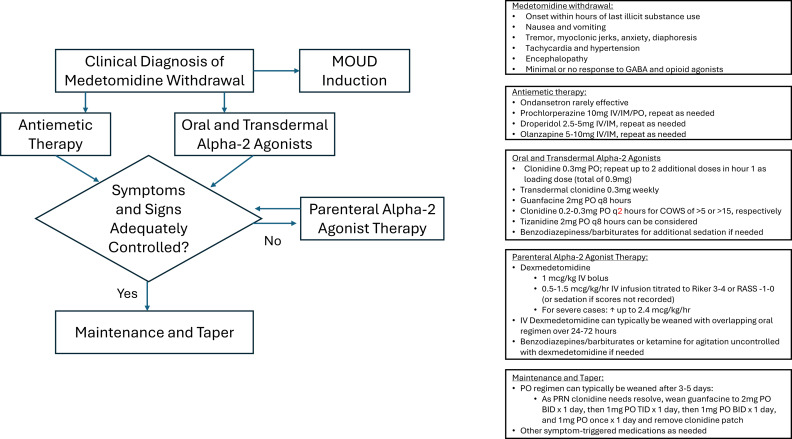
Category: Toxicology
Keywords: toxins, misperceived for edible, food containers (PubMed Search)
Posted: 2/4/2026 by Kathy Prybys, MD
Click here to contact Kathy Prybys, MD

Poisonings due to storage in a secondary container reported to the National Poison Data System, 2007–2017. Carpenter J., Murray B. et al., Clinical Toxicology, 2021.59(6), 521–527.
Poisoning following exposure to chemicals stored in mislabelled or unlabelled containers: a recipe for potential disaster. Millard YC, Slaughter RL, et al. New Zealand Med J. 26 September 2014, Vol 127 No 1403.
Unintentional poisoning from decanted toxic household chemicals. Von Fabeck K, Boulamery A, et al. Clin Toxicol (Phila). 2023 Mar;61(3):186-189.
Antifreeze on a freezing morning: ethylene glycol poisoning in a 2-year-old. Hann G, Duncan D, et al. BMJ Case Rep. 2012 Mar
Epidemiology of Accidental Poisoning Caused by Storage of Non-Food Substances in Food Containers and unmarked Bottles/Containers. Geller RJ, Kezirian R, Bangar P, Strong D, Carlson T. Children’s Hospital Central California; California Poison Control System (CPCS). https://www.tandfonline.com/doi/pdf/10.1080/15563650903076924
Category: Toxicology
Keywords: cannabinoids, liver enzymes, toxicology (PubMed Search)
Posted: 1/20/2026 by Lena Carleton, MD
(Updated: 1/26/2026)
Click here to contact Lena Carleton, MD
Consumer use of cannabidiol (CBD) products for medicinal and recreational purposes has increased in recent years. Regulatory barriers have limited randomized controlled trials examining the clinical and physiologic effects of cannabinoids in humans. This study aimed to evaluate the impact of daily cannabidiol oil use on liver enzymes and endocrine hormones in healthy adults.
In this double-blind, randomized, placebo-controlled study conducted at a clinical pharmacology unit in Wisconsin, 201 healthy adults were randomized to receive either oral CBD (2.5 mg/kg twice daily) or placebo. Laboratory testing was performed weekly.
Among participants receiving CBD (n = 151), 8 developed AST and ALT elevations greater than three times the upper limit of normal; 7 of these also had eosinophilia. No participants in the placebo group (n = 50) developed similar transaminase elevations. There were no significant differences between groups in measured endocrine hormones, including total testosterone, inhibin B, thyroid-stimulating hormone, total triiodothyronine, and free thyroxine.
Limitations included a modest sample size, unequal group sizes, and a relatively short duration of exposure and follow-up.
Key Takeaway: CBD use may be associated with elevations in AST and ALT. However, evidence remains limited, and abnormal liver enzymes should still prompt evaluation for alternative etiologies.
Florian J, Salcedo P, Burkhart K, et al. Cannabidiol and Liver Enzyme Level Elevations in Healthy Adults: A Randomized Clinical Trial. JAMA Intern Med. 2025;185(9):1070–1078. doi:10.1001/jamainternmed.2025.2366
Category: Toxicology
Keywords: Opiate use disorder, MOUD, initiation (PubMed Search)
Posted: 1/15/2026 by Robert Flint, MD
(Updated: 2/7/2026)
Click here to contact Robert Flint, MD
In this study reviewing data from the American College of Emergency Physicians’ Emergency Quality Network substance use disorder program, EDs prescribed naloxone in 27% of patients discharged after opioid overdose. Only 7% received ED administered or prescription for buprenorphine, etc. There is a lot of room for improvement in the care we provide for this subset of ED patients.
Weiner, Scott G. et al.
Annals of Emergency Medicine, Volume 0, Issue 0
Category: Toxicology
Keywords: Water beads, foreign body ingestion, gastrointestinal obstruction (PubMed Search)
Posted: 1/7/2026 by Kathy Prybys, MD
Click here to contact Kathy Prybys, MD
Water beads are a colorful, fun, popular, and widely available product found in children’s toys, stress squeeze balls, arts and crafts supplies, plant hydration products, air fresheners, and first aid ice packs.
These jelly-like small super-absorbent polymer balls are similar to the material found in diapers and absorb water expanding 100-800 percent of original size.
Pediatric ingestion is by far the most common poisoning exposure route but insertion into ears and nose and aspiration can occur and has led to serious adverse effects. More than 8000 water bead-related ingestion injuries have been treated in U.S. Emergency Departments.
Over the past 10 years, U.S. Poison Centers reported 19,660 exposures with 55% occurring in 2023 alone. In the majority of cases, no clinical effects (~88%) were seen, however in >11% of cases mild to moderate effects (abdominal discomfort, nausea, and vomiting) were reported and severe effects including complete bowel obstruction, necrosis, and surgical intervention in 0.11%. The Consumer Product Safety Commission reported at least one death of a 10-month-old girl in 2023 due to water bead ingestion.
Ingested water beads quickly pass into the small intestines where they continue to expand over the next few days and can become large enough (especially in children less than 2 year of age) to be unable to pass through the ileocecal valve causing small bowel obstruction requiring surgical intervention.
There is little data to guide management after ingestion. The majority of cases have no clinical effects and home observation is appropriate for asymptomatic for patients greater than 2 years. Recommendations from a report of case series and literature review , in patients less than 2 years of age with evidence of ingestion and symptomatic patients include hospitalization, imaging with US or CT, and close monitoring. CT, ultrasound, and endoscopy are not 100% reliable and often do not visualize these intraluminal foreign bodies.
In December 2025, the CPSC approved new federal safety standards for water beads toys setting limits on maximum expansion size of beads and amount of allowable acrylamide.
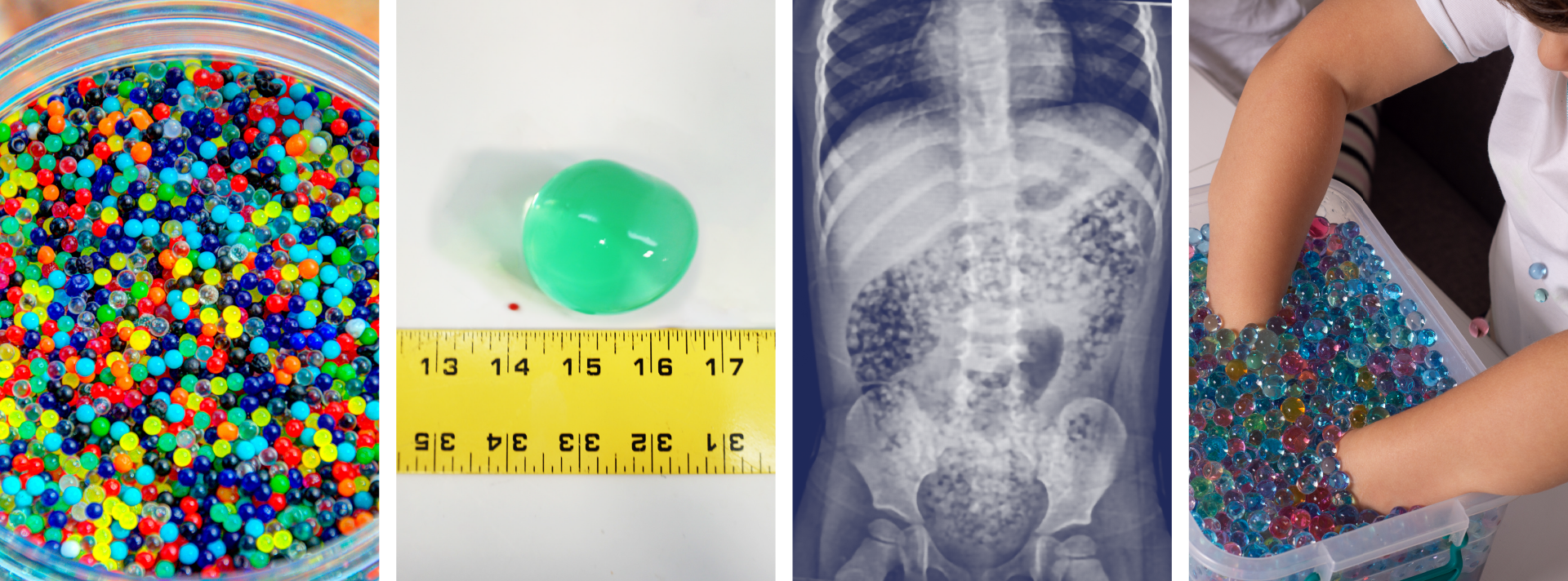
Risks of Water Bead Ingestion. Reeves PT, Pasman EA Pediatrics February 2025; 155 (2): e2024069447. 10.1542/peds.2024-069447
Water bead injuries by children presenting to emergency departments 2013-2023: An expanding issue. ?Pasman EA, Khan MA, et al. J Pediatr Gastroenterol Nutr. 2024 Sep;79(3):752-757. doi: 10.1002/jpn3.12333. Epub 2024 Jul 24. PMID: 39045753.
Pediatric water bead-related visits to United States emergency departments, Joynes HJ, Kistamgari S, et al. The American Journal of Emergency Medicine, Volume 84, 2024, Pages 81-86, ISSN 0735-6757, https://doi.org/10.1016/j.ajem.2024.07.048.
Water beads: Expanding toy and ‘new’ problem for paediatric surgeons and community. Bollettini, T., Mogiatti, M., et al. J Paediatr Child Health, 61: 204-208. 2025. https://doi.org/10.1111/jpc.16730
Aspiration of superabsorbent polymer beads resulting in focal lung damage: a case report. Alharbi N, Dabbour M. BMC Pediatr. 2020 May 29;20(1):262. doi: 10.1186/s12887-020-02168-9. PMID: 32471401; PMCID: PMC7257448.
Category: Toxicology
Posted: 12/29/2025 by TJ Gregory, MD
Click here to contact TJ Gregory, MD
Winter is in full swing and an escape to warmer climes is the only answer for many. That also means enjoying local tropical seafood and associated toxins. How do you differentiate, manage, and properly recognize the ABEM prompt?
Scombroid - HISTAMINE reaction
Ciguatera - CIGUATOXIN contaminated reef fish
Category: Toxicology
Keywords: Nitrous Oxide, Whippit, unregulated psychotropic, inhalant abuse (PubMed Search)
Posted: 12/2/2025 by Kathy Prybys, MD
(Updated: 12/3/2025)
Click here to contact Kathy Prybys, MD

Vohra V, Matthews H, Stroh-steiner G. Notes from the field: Recreational Nitrous Oxide Use-Michigan, 2019-2023. MMWR Morb Mort Wkly Rep 2025;74:210-212. DOI: http://dx.doi.org/10.15585/mmwr.mm7412a3.
Gummin D, Mowry J, Beuhler MC, et.al (17 Dec 2024): 2023 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 41st Annual Report, Clinical Toxicology, DOI: 10.1080/15563650.2024.2412423
Yockey RA, Hoopsick RA. US Nitrous Oxide Mortality. JAMA Netw Open. 2025;8(7):e2522164. doi:10.1001/jamanetworkopen.2025.22164.
https://www.fda.gov/food/alerts-advisories-safety-information/fda-advises-consumers-not-inhale-nitrous-oxide-products. FDA Advises Consumers Not to Inhale Nitrous Oxide Products 6/4/2025
Category: Toxicology
Keywords: Cyanide, antidote, hydroxycobalmin, drug shortage (PubMed Search)
Posted: 11/5/2025 by Kathy Prybys, MD
(Updated: 2/7/2026)
Click here to contact Kathy Prybys, MD
Cyanide is one of the deadliest known poisons causing immediate toxic effects and lethality within seconds to minutes. Exposures are rare, most commonly by inhalational route (HCN gas) from structural fires due to combustion of synthetic materials or from ingestion of cyanide salts. Cyanide toxicity can also occur from dermal or parental (sodium nitroprusside) exposure.
The preferred first line antidote is hydroxycobalamin (vitamin B12) available as Cyanokit, which has higher affinity for cyanide than cytochrome oxidase and binds to form harmless cyanocobalamin and is renally excreted. Limited studies reveal good survival rates in noncardiac arrest patients. Hydroxycobalamin has minimal side effects (red skin and urine, increased BP) and is well-tolerated with safer and simpler mechanism of action than Nithiodote (original antidote), containing sodium nitrite (CN preferentially binds methemoglobin to form cyanomethemoglobin) and thiosulfate (provides sulfur to convert cyanide to thiocynate for excretion). Sodium nitrite has numerous adverse effects causing hypotension and methemoglobin (contraindicated in smoke inhalation victims due to concern for carbon monoxide poisoning, G6PD deficiency, preexisting amenia), and hypersensitivity reactions. Sodium thiosulfate has less side effects and augments cyanide excretion but is considered less effective due to its slow onset, short half-life, low volume of distribution, and poor intracellular penetration.
As of August 2025, the American Society of Health -System Pharmacists (ASHP) Drug Shortage lists Cyanokit as “limited availability” in the U.S. as manufacturing was suspended due to investigation of ongoing quality defect with concern for sterility and endotoxin content. Impacted batches were released and their numbers are listed in an FDA bulletin (see references). Healthcare providers should weigh the potential benefit of using Cyanokit against the risk of infection. Infusion set with 0.2 micron in line filter can be temporarily used for administration of Cyanokit 5 mg hydroxycobalmin to prevent potential infection.
Surviving Cyanide Poisoning: A case report highlighting the role of early antidote use. Hopes BC, Slob EM, et al. Toxicology Reports, Volume 15, December 2025.
Challenges in the diagnosis of acute cyanide poisoning. Parker-Cote JL, Rizer J, et al. Clin Toxicol. 2018 Jul:56(7):609-617.
American Society of Health -System Pharmacists (ASHP) Drug Shortage Detail-Hydroxocobalmin for injection 9/22/2025.
February 6, 2025 Manufacturer letter to healthcare professional https://www.fda.gov/media/185400/download
Category: Toxicology
Keywords: Carbon Monoxide, Hyperbaric (PubMed Search)
Posted: 9/26/2025 by TJ Gregory, MD
(Updated: 9/29/2025)
Click here to contact TJ Gregory, MD
Carbon Monoxide Poisoning (COP) is a major toxicologic pathology and a common case in the Emergency Department and pre-hospital setting. History is a key component in assessment with the standard diagnostic test being blood gas analysis of Carboxyhemoglobin (COHb).
Standard pulse oximeter devices are not capable of differentiating oxyhemoglobin from carboxyhemoglobin, leading to the classic pearl that pulse ox may be falsely reassuring in COP.
In recent years, devices capable of differentiating oxyhemoglobin from COHb have been developed and are fielded in many hospitals and EMS agencies.
This meta-analysis reviews diagnostic accuracy of pulse CO-oximetry (spCO) devices in comparison to a reference standard COHb blood test. Six studies (1734 patients) were included.
This analysis found that spCO testing has a low sensitivity and high specificity.
Pooled sensitivity 0.65 (95% CI 0.44–0.81)
Pooled specificity 0.93 (95% CI 0.83–0.98)
Pooled LR+ 9.4 (95% CI 4.4 to 20.1)
Pooled LR- 0.38 (95% CI 0.24 to 0.62)
The authors conclude that the low sensitivity precludes use of spCO as an effective screening tool for COP or substitute for COHb. Conversely, we can recognize the utility of the high specificity in identifying patients who do have clinically significant toxicity. Indeed, the authors discuss potential applications for triage and transport to a hyperbaric oxygen chamber for those who are found to have elevated readings.
Technology advancement and refinement will be interesting to follow. In the meantime, don’t skip the COHb lab just because spCO measurement is reassuring.
Category: Toxicology
Keywords: alcohol withdrawal, phenobarbital, protocol, implimentation (PubMed Search)
Posted: 9/24/2025 by Robert Flint, MD
(Updated: 9/25/2025)
Click here to contact Robert Flint, MD
This study looking at pre and post-phenobarbital order set use to treat inpatient alcohol withdrawal syndrome found:
“AWS symptoms resolved more rapidly after implementation, with a 4.2- to 5.0-point reduction in daily maximum CIWA-Ar scores at 24 to 96 hours from hospital presentation, 30.1-hour reduction in AWS treatment duration (95% CI, 16.7-43.5 hours), and 2.2-day reduction in time to hospital discharge (95% CI, 0.7-3.7 days). Safety outcomes did not significantly differ before and after implementation.”
Remember phenobarbital can be used for alcohol withdrawal for our ED patients as well.
Here is the protocol:
Nursing
Vital signs 10 minutes after phenobarbital loading dose
Clinical Institute Withdrawal Assessment for Alcohol Revised (CIWA-Ar) every 1-4 hours based on score
Loading Dose
Phenobarbital 15 mg/kg intravenous piggyback (recommended for most patients)
Phenobarbital 10 mg/kg intravenous piggyback (low risk or heavily pretreated with benzodiazepines)
As-Needed Doses
Phenobarbital 130 mg intravenous twice as needed for uncontrolled agitation or CIWA-Ar ?15
Phenobarbital 260 mg intravenous once as needed for uncontrolled agitation or CIWA-Ar ?15
Wolpaw BJ, Oren H, Quinnan-Hostein L, et al. Hospital-Wide Implementation, Clinical Outcomes, and Safety of Phenobarbital for Alcohol Withdrawal. JAMA Netw Open. 2025;8(8):e2528694. doi:10.1001/jamanetworkopen.2025.28694
Category: Toxicology
Keywords: Toxicology, contaminate, opiate, stimulant (PubMed Search)
Posted: 4/5/2025 by Robert Flint, MD
(Updated: 2/7/2026)
Click here to contact Robert Flint, MD
This study from Australia reminds us that what patients think they ingested isn’t always what they did ingest. A high percentage of “cocaine” and other stimulants was actually fentanyl or other opiates. The authors do a nice job referencing similar studies in the United States. Any overdose could be a mixed picture due to impure street drugs.
Emergency Medicine AustralasiaVolume 37, Issue 2 e70038
Original Research
Open Access
Peter Chisholm MBBS, B Med Sc, MPH, Jared Brown BPharm, MPH, Thanjira Jiranantakan MD, MPH, FAFPHM, FACOEM, Mary Ellen Harrod PhD, Catherine McDonald BSc, Una Cullinan BSc, Darren M Roberts MBBS, PhD, FRACP, FAChAM
First published: 03 April 2025
Category: Toxicology
Keywords: Alcohol, mortality, predictor, trauma (PubMed Search)
Posted: 3/31/2024 by Robert Flint, MD
(Updated: 2/7/2026)
Click here to contact Robert Flint, MD
This retrospective population cohort study looked at first time ED visits for adolescents and young adults comparing those with visits related to alcohol to those not related to alcohol. Patients in the alcohol related visit group had a threefold increased one year mortality rate. Cause of death was trauma, poisoning by drug and alcohol. Risk factors include being male, age 20-29, history of mental health and having a visit for withdrawal.
Adolescents and young adults presenting to an emergency department for an alcohol related complaint are high risk for one year mortality and deserve intervention and appropriate referral.
Academic Emergency MedicineVolume 31, Issue 3 p. 220-229
Mortality in adolescents and young adults following a first presentation to the emergency department for alcohol
Lyndsay D. Harrison MSc, Asnake Y. Dumicho MSc, Anan Bader Eddeen MSc, Peter Tanuseputro MD, MHSc, Claire E. Kendall MD, PhD, Jess G. Fiedorowicz MD, PhD, Tea Rosic MD … See all authors
Category: Toxicology
Keywords: bupropion, QRS widening, NaHCO3 (PubMed Search)
Posted: 2/15/2024 by Hong Kim, MD
(Updated: 2/7/2026)
Click here to contact Hong Kim, MD
Bupropion associated cardiac toxicity widens the QRS complex by inhibiting the cardiac gap junction, not cardiac Na channel blockade. NaHCO3 is often administered when EKG changes are noted. But the effectiveness of NaHCO3 in bupropion toxicity is not well established.
A retrospective study between 2010-2020 showed, that administration of NaHCO3 only decreased QRS duration by 2 msec (median). The median NaHCO3 administered was 100 mEq. Although this study was limited by the fact that it only had a small sample size of 13, NaHCO3 administration may provide limited clinical benefit in patients with QRS widening from bupropion overdose.
Simpson M et al. Sodium bicarbonate treatment for QRS widening in bupropion overdoses
Category: Toxicology
Keywords: acetaminophen overdose, fomepizole, NAC (PubMed Search)
Posted: 7/19/2023 by Natasha Tobarran, DO
(Updated: 2/7/2026)
Click here to contact Natasha Tobarran, DO
Acetaminophen (APAP) is the leading cause of acute liver failure worldwide. Standard treatment for APAP overdose is with N-acetylcysteine (NAC), which is highly effective if given within 8 hours of ingestion. However, in delayed presenters or massive ingestions patients can still develop hepatotoxicity. Adjunctive therapies can be considered in these cases including augmented NAC dosing, renal replacement, and fomepizole.
A small amount of APAP is metabolized to N-acetyl-p-benzoquinone imine (NAPQI) by cytochrome 2E1. In therapeutic doses, the body is able to detoxify the NAPQI using glutathione. In overdose, glutathione stores get depleted and NAPQI can cause hepatotoxicity. Mitochondrial damage in APAP overdose is mediated by the c-Jun-N-terminal Kinase (JNK) pathway.
NAC works to replenish glutathione stores and detoxify NAPQI. In large overdoses, increased dosing of NAC may be necessary. Fomepizole is typically used for its alcohol dehydrogenase inhibitor property to treat methanol and ethylene glycol poisoning. Fomepizole is also a cytochrome 2E1 and JNK inhibitor and can be used in APAP overdose to block the formation of NAPQI and mitigate mitochondrial damage. Dialysis can be used to eliminate APAP from the body completely in massive overdoses or if significant acidosis or renal failure.
This study is a case series of 14 patients treated for APAP overdose between 2017 – 2021 at a tertiary hospital
Limitations of the study:
In summary:
Stephanie L. Link, Garrett Rampon, Stephen Osmon, Anthony J. Scalzo & Barry H. Rumack (2022) Fomepizole as an adjunct in acetylcysteine treated acetaminophen overdose patients: a case series, Clinical Toxicology, 60:4, 472-477, DOI: 10.1080/15563650.2021.1996591
Category: Toxicology
Keywords: CO (PubMed Search)
Posted: 7/13/2023 by Mak Moayedi, MD
(Updated: 2/7/2026)
Click here to contact Mak Moayedi, MD
Age >36
LOC
Prolonged exposure (>24hrs)
COHgb level >25%
Weaver LK, Valentine KJ, Hopkins RO. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. American journal of respiratory and critical care medicine. 2007;176(5):491-497. doi:10.1164/rccm.200701-026OC
Category: Toxicology
Keywords: cannabis exposure, pediatric, toxicity, NPDS (PubMed Search)
Posted: 7/6/2023 by Hong Kim, MD
(Updated: 2/7/2026)
Click here to contact Hong Kim, MD
Medical Cannabis is permitted in 39 states and Washington DC while 18 sates and Washington DC has legalized recreational cannabis use. As cannabis products become more available, pediatric exposure has also increased.
A retrospective study of National Poison Data System involving children < 6 years from 2017 and 2021 showed: Pre-COVID (2017-2019) & COVID (2020-2021)
Common Clinical effects
Disposition
Conclusion
Tweet MS, Nemanich A, Wahl M. Pediatric Edible Cannabis Exposures and Acute Toxicity: 2017–2021. Pediatrics. 2023;151(2):e2022057761
Category: Toxicology
Keywords: Lithium, Lab error, Toxicity (PubMed Search)
Posted: 6/15/2023 by Natasha Tobarran, DO
(Updated: 2/7/2026)
Click here to contact Natasha Tobarran, DO
Lithium toxicity can present acutely with gastrointestinal symptoms and chronically with neurologic symptoms such as tremor and ataxia. Diagnosis and treatment with normal saline hydration and/or dialysis depends on lithium levels in conjunction with signs and symptoms.
Lithium levels can be falsely elevated when blood samples are collected in green top tubes which contain lithium heparin, or if the blood collection volume is too small. Not recognizing that a lithium level may be falsely elevated can lead to misdiagnosis as well as unnecessary hospitalizations and treatments. The study by Wills et al found lithium levels as high as 4 mmol/L (therapeutic range 0.6-1.2 mmol/L) in lithium naïve volunteers collected in the wrong tube and with small blood volumes. If a patient has an elevated lithium level in the absence of lithium toxicity symptoms, consider a falsely elevated level and redraw using the appropriate tube and sample size.
In summary:
Wills BK, Mycyk MB, Mazor S, Zell-Kanter M, Brace L, Erickson T. Factitious lithium toxicity secondary to lithium heparin-containing blood tubes. J Med Toxicol. 2006 Jun;2(2):61-3. doi: 10.1007/BF03161172. PMID: 18072115; PMCID: PMC3550057.
Category: Toxicology
Keywords: amlodipine, non-dihydropyridines, high-dose insulin (PubMed Search)
Posted: 6/1/2023 by Hong Kim, MD
(Updated: 2/7/2026)
Click here to contact Hong Kim, MD
Calcium channel blocker (CCB) overdose can lead to severe shock/hypotension. A small study was conducted to compare the hemodynamic effects of high-dose insulin (HDI) for two classes of CCB (dihydropyridines vs. non-dihydropyridines) that work differently to manage hypertension.
Study design:
Study sample:
Result
Median number of maximum concomitant vasopressors (p=0.04)
Median difference in max concomitant vasopressors: 1 (95% CI: 0 – 2)
Median max epinephrine dosing
Use of rescue methylene blue (p=0.009)
Conclusion:
Cole JB, Lee SC, Prekker ME, Kunzler NM, Considine KA, Driver BE, Puskarich MA, Olives TD. Vasodilation in patients with calcium channel blocker poisoning treated with high-dose insulin: a comparison of amlodipine versus non-dihydropyridines. Clin Toxicol (Phila). 2022 Nov;60(11):1205-1213. doi: 10.1080/15563650.2022.2131565. Epub 2022 Oct 25. PMID: 36282196.
Category: Toxicology
Keywords: flumazenil, benzodiazepine overdose, adverse events (PubMed Search)
Posted: 1/13/2022 by Hong Kim, MD
Click here to contact Hong Kim, MD
Flumazenil is a reversal agent for benzodiazepine overdose. Adverse events including seizure, agitation and cardiac arrhythmias have been reported but the frequency of adverse events is unknown.
AE and serious AEs were defined as:
AE:
Serious AE (SAE):
A systematic review/meta-analyses of 13 randomized controlled trials showed
Most common AEs
Most common SAEs
Conclusion
PENNINGA E ET AL.Adverse Events Associated with Flumazenil Treatment for the Management of Suspected Benzodiazepine Intoxication--A Systematic Review with Meta-Analyses of Randomized Trials. Basic Clin Pharmacol Toxicol. 2016
DOI: 10.1111/bcpt.12434
Category: Toxicology
Keywords: xylazine, adulterate, heroin, fentanyl (PubMed Search)
Posted: 12/16/2021 by Hong Kim, MD
Click here to contact Hong Kim, MD
Xylazine is a central alpha-2 agonist (similar to clonidine) that is used as a veterinary tranquilizer. It also possesses analgesic, and muscle relaxant properties. Heroin/fentanyl is increasingly being adulterated with xylazine and resulting in severe adverse effects (CNS and respiratory depression, bradycardia, and hypotension), including deaths.
According to CDC, 0.1%-5.5% of IMF death in US between 2019 – 2020 involved xylazine.
In Philadelphia, PA:
The detection of xylazine in unintentional overdose death increased from
Approximately 25% of drug seizures in Philadelphia contained xylazine in 2019
There is no effective pharmacologic agent for xylazine toxicity. Similar to clonidine toxicity, high dose naloxone may be tried. But pediatric data show that approximately 50% of pediatric clonidine toxicity response to high-dose naloxone administration. Thus, naloxone administration may not reverse the CNS/respiratory depression, bradycardia and hypotension.
Conclusion
O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and Characteristics of Drug Overdose Deaths Involving Illicitly Manufactured Fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep 2021;70:1740-1746. DOI: http://dx.doi.org/10.15585/mmwr.mm7050e3external icon.
Johnson J, et al. Inj Prev 2021;27:395–398. doi:10.1136/injuryprev-2020-043968
