Category: Toxicology
Posted: 6/5/2015 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Electronic cigarettes are battery-powered devices that deliver nicotine, flavorings, (e.g. fruit, mint, and chocolate), and other chemicals via an inhaled aerosol. E-cigarettes are currently not regulated by the FDA. In many states, there are no restrictions on the sale of e-cigarettes to minors.
Electronic cigarette exposures involving young children are rapidly increasing. Such exposures tend to involve patients aged < 5 years and occur by ingestion of the nicotine-containing liquid. There is a potential for acute nicotine toxicity (nausea, vomiting, pallor, diaphoresis, tachycardia, hypertenstion initially). Respiratory muscle weakness with respiratory arrest is the most likely cause of death.
To date, the overwhelming majority of pediatric ingestions have not resulted in serious medical outcomes. The most commonly reported adverse events were nausea and vomiting.
However, in May of 2014, the first pediatric case of toxicity from ingestion of e-cigarette nicotine liquid was reported. A 10-month old ingested an unknown amount of e-liquid and developed vomiting, tachycardia, grunting respirations, and ataxia. The symptoms resolved by 6 hours after ingestion without specific treatment.
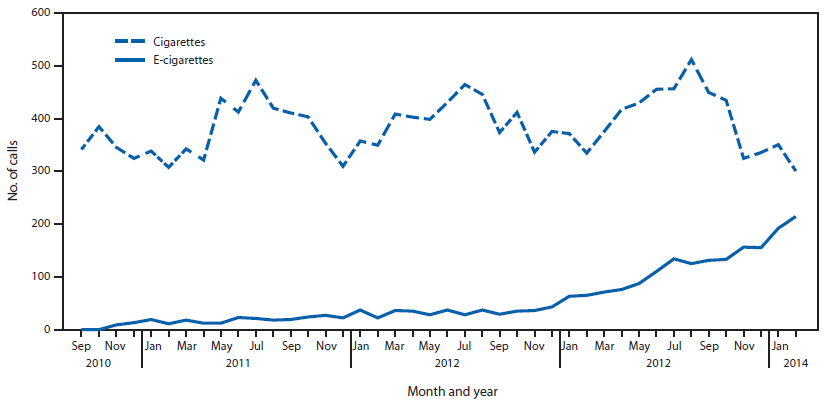
(1) The figure above shows the number of calls to poison centers for cigarette or e-cigarette exposures, by month, in the United States during September 2010 February 2014. E-cigarette exposure calls per month increased from one in September 2010 to 215 in February 2014.
(1) Chatham-Stephens K, Law R, Taylor E, et al. MMWR Morb Mortal Wkly Rep 2014;63:292-293.
(2) LoVecchio F, Zoph O. Incidence of electronic cigarette exposure in children skyrockets in Arizona. Am J Emerg Med, epub, 2/25/15.
(3) Bassett RA, Osterhoudt K, Brabazon T. Nicotine Poisoning in an Infant. N Engl J Med 2014;370:2249-2250.
Category: Toxicology
Keywords: Dabigatran, Hemodialysis, Renal Replacement Therapy (PubMed Search)
Posted: 5/14/2015 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Dabigatran is an orally administered, potent, direct thrombin inhibitor approved for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, and for the treatment and secondary prevention of venous thromboembolism.
Several pharmacokinectic studies have suggested that dabigatran possesses a number of ideal properties for expedited removal via extracorporeal methods. Dabigatran has low oral bioavailability (3-7%) and is predominantly cleared (80%) by the kidneys. It is not significantly protein bound, low-to-moderate steady state volume of distribution, and has a low molecular weight. All of these attributes make it a candidate for extracorporeal removal. Low protein binding may suggest redistribution into the plasma post extracorporeal removal.
Dabigatran is a substrate for the multidrug efflux transporter P-glycoprotein. Administration of the drug with potent P-glycoprotein inhibitors (ketoconazole, verapamil, amiodarone, quinidine) may significantly increase risk of toxicity, i.e. bleeding.
Most of the current evidence is based on case reports/case series where HD was the primary mode of removal.
Caution: Redistribution effect in plasma dabigatran concentration was also observed in several cases within 20 min to 12 hours post cessation of renal replacement therapy. Other limitations include:
1) Hemodynamic instability such as hypotension that may make initiation of extracoporeal removal difficult
2) Availability of indicators demonstrating effectiveness of extracorporeal removal
3) Amount of time needed to prepare patient to receive extracorporeal therapy
4) Use of extracorporeal removal as a treatment modality has not been prospectively evaluated
Bottom line: Extracorporeal removal may be an option for patients in the setting of life-threatening bleeding but with consideration of several limitations and should not preclude or delay use of other supplemental hemostatic therapies.
Awad NI, Brunetti L, Juurlink DN. Enhanced Elimination of Dabigatran Through Extracorporeal Methods. J Med Toxicol 2015;11:85-95.
Category: Toxicology
Keywords: Drug Screens, Drug Intervals (PubMed Search)
Posted: 2/5/2015 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
| Performance Characteristics of Common Drug Abuse Screening Immunoassays | ||
| Drug/Class | Detection Interval (***) | Comments |
| Amphetamines | 1-2 days (2-4 days) | Decongestants, ephedrine,l-methamphetamine, selegilene & bupropion metabolites may give False (+) results; MDA & MDMA are variably detected |
| Barbiturates | 2-4 days | Phenobarbital may be detected for up to 4 weeks |
| Benzodiazepines | 1-30 days | Benzos vary in reactivityand potency; False (+) results may be seen with oxaprozin |
| Cannabinoids | 1-3 days (>1 month) | Screening assays detect inactive and active cannabinoids; Confirmatory assays detects inactive metabolite THCA (tetrahydrocannabinoic acid) |
| Cocaine | 2 days (1 week) | Screening & confirmatory assays detect inactive metabolite BE (benzoylecgonine); False (+) results are unlikely |
| Opiates | 1-2 days; 2-4 days (<1 week) | Semisynthetic opiates derived from morphine show variable cross-reactivity; Fully synthetic opioids (e.g., fentanyl, meperidine, methadone, propoxyphene, tramadol) have minimal cross reactivity; Quinolone may cross-react |
| Methadone | 1-4 days | Doxylamine may cross-react |
| Phencyclidine | 4-7 days (>1 month) | Dextromethorphan, diphenhydramine, ketamine, & venlafaxine may cross react |
| Propoxyphene | 3-10 days | Duration of positivity depends on cross reactivity of metabolite norpropoxyphene |
(***)Values are after typical use; values in parentheses are after heavy or prolonged use.
Adapted from Goldfrank's Toxicologic Emergencies, 9th ed; Table 6-10.
Category: Toxicology
Keywords: Sympathomimetic toxicity, Synthetic cathinones, Rhabdomyolysis (PubMed Search)
Posted: 12/4/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Sympathomimetic toxicity is a known toxidrome that is complicated by the development of rhabdomyolysis. There are multiple stimulant agents that induce sympathomimetic toxicity including, synthetic cathinones, cocaine, amphetamines, and methamphetamines.
A recent retrospective, single-center, chart review in the age range of 14-65 years sought to determine the prevalence of rhabdomyolysis in patients with sympathomimetic toxicity and compare rates among patients using specific agents. Rhabdomyolysis and severe rhabdomyolysis were defined as CK>1000 and 10,000 IU/L, respectively.
Rhabdomyolysis occurred in 42% of study subjects (43/102)
Prevalence in 89 subjects due to a single-stimulant exposure:
Rhabdomyolysis
1) Synthetic cathinone (MDPV, alpha-PVP) 63% (12/19)
2) Methamphetamine 40% (22/55)
3) Cocaine 33% (3/9)
4) Other single agents (methylphenidate, pseudoephedrine, phentermine) 0% (0/6)
Severe Rhabdomyolysis
1) Synthetic cathinone 26% (5/19)
2) Methamphetamine 3.6% (2/55)
3) Cocaine 11% (1/9)
4) Other single agents (methylphenidate, pseudoephedrine, phentermine) 0% (0/6)
In this study, patients exposed to synthetic cathinones were more likely to develop rhabdomyolysis and severe rhabdomyolysis compared to the non-cathinone-exposed group.
Bottom Line:
Be aware of this increased risk from synthetic cathinones along with other stimulants. Treat aggressively with IV fluids, rapid correction of hyperthermia, benzodiazepines to control manifestations of sympathomimetic toxicity to reduce muscle activity and metabolic demand.
O'Connor AD, Padilla-Jones A, Gerkin AD, et al. Prevalence of Rhabdomyolysis in Sympathomimetic Toxicity: a Comparison of Stimulants. J Med Toxicol 2014; Dec 3; Epub ahead of print.
Category: Toxicology
Keywords: Digoxin, Cardioactive Steroids, Digitoxin, Digoxin-specific Fab Fragments (PubMed Search)
Posted: 11/7/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Digoxin-specific antibodies are produced in immunized sheep and have high binding affinity for digoxin and, to a lesser extent, digitoxin and other cardiac glycosides. The Fab fragment binds free digoxin and once the digoxin-Fab complex is formed, the digoxin molecule is no longer pharmacologically active. The complex is renally eliminated and has a half-life of 14-20 hours (may increase 10-fold with renal impairment). Reversal of signs of digoxin/digitalis intoxication usually occurs within 30-60 minutes, with complete reversal varying up to 24 hours.
Contraindication: None known. Caution is warranted in patients with known sensitivity ot ovine (sheep) products. Product may contain traces of papain and caution advised in patients with allergies to papain, papaya extracts, chymopapain.
Adverse effects:
1) Monitor for potential hypersensitivity reactions and serum sickness
2) In patients with renal insufficiency and impaired renal clearance of dig-Fab complex, a delayed rebound of free serum digoxin levels may occur
3) Removal of the effect of digoxin/digitalis may exacerbate preexisting heart failure
4) Removal of digoxin/digitalis effect may cause hypokalemia
Laboratory interaction: Digoxin-Fab complex cross-reacts with the antibody commonly utilized in quantitative immunoassay techniques. This results in falsely high serum concentrations of digoxin due to measurement of the inactive Fab complex. Therefore, measure free digoxin levels, which may be useful for patients with renal impairment.
Dosing: Each vial of Fab product binds 0.5 mg of digoxin.
Digoxin-specific Fab (round up vial calculation)
# of vials = Digoxin concentration (ng/mL) x Pt Wt (kg)
100
Category: Toxicology
Keywords: Digoxin, Cardioactive Steroids, Digitoxin, Digoxin-specific Fab Fragment (PubMed Search)
Posted: 10/1/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Cardioactive steroids are among the many treatments used for CHF, and for the control of ventricular response rate in atrial tachydysrhythmias. There are many sources of cardioactive steroids:
Pharmaceutial: Digoxin, Digitoxin
Plants: Oleander, Yellow Oleander, Foxglove, Lily of the Valley, Dogbane, Red Squill
Animal: Bufo marinus toad
It is a potent Na+-K+-ATPase inhibitor and can lead to hyperkalemia in acute ingestion with associated signs and symptoms of N/V, abdominal pain, bradycardia and possibly, hypotension.
Toxicity should be suspected with bidirectional ventricular tachycardia or atrial tachycardia with high-degree AV block
Therapeutic range of digoxin of 0.5 - 2.0 ng/mL is helpful but not a sole indicator of toxicity
Indication for antidote (Digoxin-specific Antibody Fragments) include:
1) Digoxin-related life-threatening dysrhythma
2) Serum K+ > 5.0 mEq/L in acute ingestion
3) Serum digoxin concentration >15ng/mL at any time, or >10 ng/mL 6 hours postingestion
4) Ingestion of 10 mg in adult; 4 mg in pediatric
5) Poisoning by non-digoxin cardioactive steroid
Goldfrank's Toxicologic Emergencies, 9th edition
Category: Toxicology
Keywords: Halogenated hydrocarbons, cardiac sensitization (PubMed Search)
Posted: 9/4/2014 by Kishan Kapadia, DO
(Updated: 2/8/2026)
Click here to contact Kishan Kapadia, DO
Dysrhythmia-induced sudden death, termed "sudden sniffing death syndrome," is well described phenomena due to inhalant (chlorinated and aromatic hydrocarbon) abuse.
Common inhalants include:
Chlorinated hydrocarbons: Degreasers, spot removers, dry-cleaning agents
Fluorocarbons: Freon gas, deodarants
Toluene: Paint thinners, spray paint, airplane glue
Butane: Lighter fluid, fuel
Acetone: Nail polish remover
The common theory behind the syndrome is cardiac sensitization that increases susceptibility of the heart to systemic catecholamines (epinephrine, norepinephrine, etc). Usually, it occurs after an episode of exertion in that any excess catecholamine exposure causes irritability of the myocardium, resulting in dysrhythmias (V. fib, V. tach) and cardiac arrest.
If acute dysrhythmias is due to myocardial sensitization, sympathomimectis should be avoided. Beta-adrenergic antagonist can be used for the catecholamine-sensitized heart.
Category: Toxicology
Keywords: Bath salts, mephedrone, agitated delirium (PubMed Search)
Posted: 7/31/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Bath salts (synthetic cathinones) commonly contain multipe synthetic drugs and can be ingested, smoked, or administered intravenously. The designer stimulant mephedrone (4-methylcathinone) is among the most popular of the derivatives of the naturally occurring psychostimulant cathinone. Bath salt use is on the rise and is responsible for a large number of ED visits.
In spite of their ban, bath salts are still available over the counter in specialty shops and through the Internet with common product names such as: "Ivory Wave," "Cloud 9," "Purple Wars," "Vanilla Sky," "Bliss," etc. They are commonly marketed with the disclaimer, "not for human consumption."
Their presentation mimics other sympathetic drugs through pathways similar to amphetamines. The primary psychological effects have a duration of roughly 3-4 hours, with physiologic effects lasting from 6-8 hours.
| Physical Effects | Behavioral & Mental Status Effects |
| Tachycardia | Agitation |
| Hypertension | Paranoia |
| Dysrhythmias | Hallucinations |
| Hyperthermia | Psychosis |
| Seizures | Violent behavior |
| Sweating | Delusions |
Management is largely supportive and includes IV hydration, benzodiazepines, and close monitoring in the ICU setting.
Imam SF, Patel H, Mahmoud M, et.al. Bath salts intoxication: A case series. JEM 2013;45(3):361-365.
Category: Toxicology
Keywords: Colchicine, Poisoning, Arrhythmia (PubMed Search)
Posted: 6/29/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Colchicine tablets and injectable solution is frequently used for the treatment of gout and familial Mediterranean fever. An overdose is extremely serious, with considerable mortality that is often delayed. It is considered a cellular poison due to its inhibition of cellular mitosis of dividing cells.
After an acute overdose, symptoms typically are delayed for 2-12 hours and include nausea, vomiting, abdominal pain, and severe bloody diarrhea.
Chronic poisoning presens with a more insidious onset.
Late complications include bone marrow suppression, particularly leukopenia and thrombocytopenia (4-5 days) and alopecia (2-3 weeks).
Treatment includes aggressive supportive care, monitoring and treatment of fluid and electrolyte disturbances.
The usual cause of death from acute poisoning is due to hemodynamic collapse and cardiac arrhythmias (typically 24-36 hours after ingestion or could be sudden) or from infectious or hemorrhagic complications.
1) Finkelstein Y et al. Colchicine poisoning: the dark side of an ancient drug. 2010 Clin Tox 48(5):404-414.
2) Olson KR, ed. Poisoning & Drug Overdose. 5th ed. New York: McGraw Hill; 2007.
Category: Toxicology
Keywords: Methemoglobenima, methylene blue, adverse effects (PubMed Search)
Posted: 5/21/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Methylene blue is an extremely effective antidote for acquired methemoglobinemia but has important adverse effects if given in excess of recommended dose.
Below is the usual dose of methylene blue for treatment of methemoglobinemia
1-2 mg/kg of 1% solution IV with a repeat dose given if there is inadequate response to the first one
Adverse effects include:
Lo, J et al. A review of methylene blue treatment for cardiovascular collapse. 2014 J Emerg Med 46(5):670-679.
Category: Toxicology
Keywords: Envenomation, Compartment Syndrome, Risk Factors (PubMed Search)
Posted: 4/24/2014 by Kishan Kapadia, DO
Click here to contact Kishan Kapadia, DO
Venomous snakes are believed to be everywhere in the United States except Maine, Hawaii, and Alaska. Most snakebites occur from months of April to October since snakes hibernate in the winter. Most bites occur in the extremities (lower > upper). One of the serious clinical manifestation of snakebite is compartment syndrome.
The following are risk factors for the development of increased intracompartmental pressures:
1) Envenomation of small children
2) Envenomation of digits
3) Application of ice or cold packs
4) Delayed use of antivenin
5) Inadequate dosing of antivenin
Cumpston KL. Is there a role for fasciotomy in Crotalinae envenomations in North America? 2011. Clin Tox 49(5):351-365.