Category: Critical Care
Posted: 1/10/2024 by William Teeter, MD
Click here to contact William Teeter, MD
Many of us in the endovascular resuscitation space were eagerly awaiting some clarity on REBOA from this trial. Unfortunately, this is not the definitive trial that either confirms or denies the utility of REBOA in trauma.
Unfortunately, even this well-designed trial suffered from major problems, most notably enrollment issues (ITT: of the 46 in the REBOA group, only 19 actually got REBOA!!) and matching issues (Brain AIS was significantly higher in the REBOA group versus standard practice [3 vs 0] & initial systolic pressure was lower in the REBOA group, both of which are known risk factors for poor outcome in REBOA).
This trial's failure to provide a definitive benefit or the nail-in-the-coffin is frustrating to say the least. Until that day, we will continue to be selective of the "right" patient and to put in femoral arterial lines early and often.
Zaf Qasim has an excellent talk on EMRAP about this study, as does St. Emlyn's.
Category: Critical Care
Posted: 1/2/2024 by Mark Sutherland, MD
Click here to contact Mark Sutherland, MD
As is well known, fluid resuscitation strategy ("liberal" vs “restrictive”) in sepsis is a controversial topic. An RCT in NEJM called CLOVERS that looked at this and found no difference was recently re-analyzed to answer the following question… should my choice of strategy change if the patient presents with an Acute Kidney Injury (AKI)?
For the most part, the answer is no. In the group with AKI, the restrictive group did slightly, but non-statistically-significantly, better. Interestingly, in the group without AKI, the relationship reversed, and in fact of the 4 groups (AKI vs no AKI, Restrictive vs Liberal), the no AKI but liberal strategy group did best (liberal vs restrictive in the no AKI group almost reached statistical significance in favor of the liberal strategy, but not quite).
Bottom Line: In septic patients presenting with an AKI, we don't know whether liberal or restrictive strategy is better, but either is probably reasonable. In patients presenting without an AKI, it may be more ok to lean more towards liberal fluid resuscitation than in non-AKI patients*.
*There are several important caveats here: 1) they didn't closely evaluate for potential side effects of over-resuscitation such as hypoxia or pulmonary edema (the primary outcome was need for renal replacement therapy), 2) as mentioned above, this trended towards but did not reach statistical significance, 3) this is one small study which did a subgroup secondary-analysis of a larger trial.
Category: Critical Care
Posted: 12/19/2023 by Mike Winters, MBA, MD
(Updated: 2/9/2026)
Click here to contact Mike Winters, MBA, MD
Acute-On-Chronic Liver Failure
Perricone G, et al. Intensive care management of acute-on-chronic liver failure. Critical Care. 2023;49:903-21.
Category: Critical Care
Keywords: Critical Care, Burn, Resuscitation (PubMed Search)
Posted: 12/13/2023 by Lucas Sjeklocha, MD
Click here to contact Lucas Sjeklocha, MD
Bottom line: In the 2023 updated Clinical Practice Guideline, the American Burn Association recommends 2ml/kg/%TBSA (for burns >20% TBSA)as initial starting point for fluid administration in the first 48 hours, guided by clinical factors with consideration of supplemental albumin to limit fluid administration. Fresh frozen plasma should be considered in the context of a clinical trial. Vitamin C and advanced hemodynamic monitoring are not recommended as they have not demonstrated improved outcomes.
Summary: Burn care has a paucity of high-quality research about some of the fundamental questions for resuscitation. The American Burn Association since 2010 has endorsed fluid volumes for patients with >20% TBSA (i.e. those predicted to develop burn shock) from 2ml/kg/%TBSA to 4ml/kg/%TBSA as a starting point for fluid resuscitation. Further clinical studies since then have demonstrated that lower volumes of fluid targeting urine output and other physiological variables are effective without demonstrating clear improvement in patient centered outcomes. Further adjuncts such as albumin or fresh frozen plasma have demonstrated reduced fluid administration but no improvement in patient-centered outcomes. While “fluid creep” is increasingly recognized, demonstrating benefits in clinical trials will likely remain elusive as overall practice continues to shift towards less fluids and the adjunctive use of colloid will likely continue to expand. In addition to ABA CPGs and resources, the Joint Trauma System also has several useful resources for burn care.
Sources:
https://doi.org/10.1093/jbcr/irad125
https://jts.health.mil/assets/docs/cpgs/Burn_Care_11_May_2016_ID12.pdf
Category: Critical Care
Keywords: vasopressor, norepinephrine, timing, septic shock (PubMed Search)
Posted: 12/5/2023 by Quincy Tran, MD, PhD
(Updated: 2/9/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: systemic review and meta-analysis
Participants: 2 RCTs, 21 observational studies. Fifteen studies were published between 2020-2023.
There was a total of 25721 patients with septic shock
Outcome measurement: Primary outcome was short-term mortality (ICU, hospital, 28-day, 30-day). Secondary outcomes included ICU LOS, Hospital LOS, time to achieve MAP > 65 mm Hg,
Study Results:
Composite outcome of short term mortality:
Secondary outcome:
Discussion:
Conclusion:
More and more studies, although a RCT is still necessary, are showing that early initiation of vasopressor within 1-6 hours of septic shock would be more beneficial to patients with septic shock.
Ye E, Ye H, Wang S, Fang X. INITIATION TIMING OF VASOPRESSOR IN PATIENTS WITH SEPTIC SHOCK: A SYSTEMATIC REVIEW AND META-ANALYSIS. Shock. 2023 Nov 1;60(5):627-636. doi: 10.1097/SHK.0000000000002214. Epub 2023 Sep 2. PMID: 37695641.
Category: Critical Care
Posted: 11/28/2023 by Caleb Chan, MD
(Updated: 2/9/2026)
Click here to contact Caleb Chan, MD
McCallister R, Nuppnau M, Sjoding MW, Dickson RP, Chanderraj R. In patients with sepsis, initial lactate clearance is confounded highly by comorbidities and poorly predicts subsequent lactate trajectory. CHEST. 2023;164(3):667-669.
Category: Critical Care
Posted: 11/23/2023 by William Teeter, MD
Click here to contact William Teeter, MD
https://pubmed.ncbi.nlm.nih.gov/37142091/
Category: Critical Care
Keywords: Pneumonia, Corticosteroids, Steroids, Respiratory Failure, Infection (PubMed Search)
Posted: 11/9/2023 by Mark Sutherland, MD
Click here to contact Mark Sutherland, MD
For the folks who have been in practice for a while, you may be aware of the roller-coaster evidence base looking at steroids for pneumonia. Once thought to be beneficial and clearly indicated, of late steroids for pneumonia have fallen out of favor. Hamad et al have published an excellent (and brief) review in Clinical Infectious Diseases which suggests the pendulum might be swinging back in favor of giving steroids to patients with pneumonia. It's a ~5 minute read, so I recommend glancing through it yourself, but below are my two cents (solely my opinion) on where we are with steroids for pneumonia.
Take Home Points (OPINION ALERT):
1) When you have a condition present that you consider an indication for steroids (e.g. severe COVID-19 for sure; septic shock, s. pneumo infection, and ARDS depending on how you feel about the existing literature) --> strongly consider giving steroids unless there's a contraindication
2) When you have an undifferentiated patient who MAY have one of these conditions (e.g. pneumonia with COVID pending, patient potentially in ARDS or high risk of going into ARDS, etc) who is very sick --> it is reasonable to give steroids (if no contraindication) or not give steroids. My tendency is to lean towards giving steroids in these cases, but do be aware that society guidelines recommend against steroids here (although debatable if they just haven't caught up to more recent literature)
3) When you have an undifferentiated patient who may have one of these conditions, but is NOT very sick --> I do not think there is significant enough evidence to support empiric steroids
4) Factors that might push you one way or another:
Category: Critical Care
Posted: 10/31/2023 by Mike Winters, MBA, MD
(Updated: 2/9/2026)
Click here to contact Mike Winters, MBA, MD
IV Fluid Resuscitation
Kaufman DA, et al. The ins and outs of IV fluids in hemodynamic resucitation. Crit Care Med. 2023;51:1397-1406.
Category: Critical Care
Keywords: SOFA, admission unit, ICU, IMC, Ward, morality (PubMed Search)
Posted: 10/17/2023 by Quincy Tran, MD, PhD
(Updated: 2/9/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: Retrospective study of a national inpatient database (Japan).
Participants:
Outcome measurement: Primary outcome was in-hospital mortality, after propensity score matching.
Study Results:
Discussion:
Conclusion:
Risk-stratifying patients according to SOFA score is a potential strategy for appropriate admission strategies.
1.Ohbe H, Sasabuchi Y, Doi K, Matsui H, Yasunaga H. Association Between Levels of Intensive Care and In-Hospital Mortality in Patients Hospitalized for Sepsis Stratified by Sequential Organ Failure Assessment Scores. Crit Care Med. 2023 Sep 1;51(9):1138-1147. doi: 10.1097/CCM.0000000000005886. Epub 2023 Apr 28. PMID: 37114933.
2.Corwin GS, Mills PD, Shanawani H, Hemphill RR. Root Cause Analysis of ICU Adverse Events in the Veterans Health Administration. Jt Comm J Qual Patient Saf. 2017 Nov;43(11):580-590. doi: 10.1016/j.jcjq.2017.04.009. Epub 2017 Jul 25. PMID: 29056178.
Category: Critical Care
Keywords: peripheral pressors, central line, CVC, CLABSI (PubMed Search)
Posted: 10/4/2023 by William Teeter, MD
Click here to contact William Teeter, MD
Bottom line: As part of a systematic protocol, peripheral pressors administered through a peripheral line greater 22Ga or larger reduced the number of days of central venous catheter (CVC) use in a MICU population at an academic medical center. 35 (5.5%) patients had an extravasation event all with “minimal” tissue injury complications. None required surgery. 51.6% of patients did not require a CVC as a result of the protocol
Details
Notes on protocol
PIV were placed and confirmed with US, were between wrist and AC fossa with q2h patency checks. Max allowable dose of NE 15 mcg/min with requirement that patients be able to report pain at site. Initially, max infusion time was set at 48h but was eventually liberalized to indefinite use.
https://pubmed.ncbi.nlm.nih.gov/37611862/
Category: Critical Care
Keywords: BRASH, shock, av nodal blockers (PubMed Search)
Posted: 9/20/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The BRASH syndrome (Bradycardia, Renal failure, AV nodal blockade, Shock, Hyperkalemia) has been increasingly described in the literature in the past 3-5 years.
The inciting factor is generally considered to be something that prompts acute kidney injury, often hypovolemia of some sort. Rather than AV nodal blocker overdose or severe hyperkalemia causing conduction problems, the combination of AV nodal blocker use (most often beta-blockers, but can be any type) and hyperkalemia (often only moderate) has a synergistic effect on cardiac conduction with ensuing bradycardia that can devolve into a cycle of worsening renal perfusion and shock.
Treatment is supportive, but most effective when the syndrome is recognized and all parts simultaneously managed. ED physicians should be familiar with its existence for targeted whole-syndrome stabilization and to avoid diagnostic delay.
Category: Critical Care
Keywords: NIPPV, CPAP, HFNC, High Flow, Respiratory Failure (PubMed Search)
Posted: 9/12/2023 by Mark Sutherland, MD
Click here to contact Mark Sutherland, MD
When patients fail simple respiratory support therapies like nasal cannula or non-rebreather, it is often a point of debate whether to move next to High Flow Nasal Cannula (HFNC) or Noninvasive Positive Pressure Ventilation (NIPPV). This study randomized patients in acute respiratory failure (ARF) to CPAP, a form of NIPPV, vs HFNC. They looked at all comers in ARF, and primary outcome was need for intubation. Importantly, they excluded asthma/COPD exacerbation, for which BiPAP is typically considered the first line therapy due to improved CO2 clearance.
They found a significantly lower number of patients required intubation in the CPAP (28.9%) group than the HFNC (42.6%) group (p=0.006). They hypothesized that the enhanced PEEP improved oxygenation (hypoxia being a common trigger for moving to intubation), but as opposed to BiPAP, the lack of additional driving pressure limited tidal volumes and Patient Self-Inflicted Lung Injury (P-SILI), which is a known mechanism of ARDS and mortality. They use this argument to explain why trials like FLORALI, pitting HFNC vs BiPAP, tend to not find an advantage for the NIPPV arm. While this rationale makes sense, it should be noted that the study does not directly investigate if this was the reason for the difference, and for what its worth the inverse argument that using driving pressure to reduce respiratory rate, hypercarbia, and work of breathing (other very common indications for intubation) would also theoretically reduce intubations. Furthermore, it's not clear why reducing P-SILI, which tends to cause mortality on a much longer duration, would improve the short-term outcome of need for intubation.
Bottom Line: This study demonstrated a benefit to CPAP over HFNC in terms of decreasing need for intubation amongst non-asthma/non-COPD patients with acute respiratory failure, and offered a physiologic rationale but one that requires further verification and discussion. While it may be reasonable to choose CPAP instead of HFNC in marginal patients at risk of intubation (but stable enough to trial noninvasive support first), in my opinion more studies are likely needed before a wholesale change in practice. The study also does not take into consideration the enhanced comfort and compliance we tend to see with HFNC over NIPPV, which should be considered as well.
Nagata K, Yokoyama T, Tsugitomi R, Nakashima H, Kuraishi H, Ohshimo S, Mori Y, Sakuraya M, Kagami R, Tanigawa M, Tobino K, Kamo T, Kadowaki T, Koga Y, Ogata Y, Nishimura N, Kondoh Y, Taniuchi S, Shintani A, Tomii K; JaNP-Hi Study Investigators. Continuous positive airway pressure versus high-flow nasal cannula oxygen therapy for acute hypoxemic respiratory failure: A randomized controlled trial. Respirology. 2023 Aug 30. doi: 10.1111/resp.14588. Epub ahead of print. PMID: 37648252.
Category: Critical Care
Posted: 9/5/2023 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Pearls for the Patient in Cardiogenic Shock
Jentzer JC, et al. Advances in the management of cardiogenic shock. Crit Care Med. 2023; 51:1222-1233.
Category: Critical Care
Keywords: arterial cannulation, axillary artery, femoral artery, infraclavicular (PubMed Search)
Posted: 8/21/2023 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Settings: Single ICU in Poland, randomized trial
Participants: intubated patients who needed arterial catheter placement. Patients who had adequate access to one axillary and one femoral artery were eligible.
Patients were randomized 1:1 for axillary or femoral artery cannulation.
Outcome measurement: Primary outcome was cannulation success rate. Secondary outcomes were first pass success rate, number of attempts.
Study Results:
Discussion:
Conclusion:
Ultrasound-guided cannulation of the axillary artery via the infraclavicular route is non-inferior to the cannulation of the common femoral artery. When cannulation of the radial or femoral artery is not available, we can consider axillary artery via the infraclavicular approach.
Reference:
Gawda, Ryszard MD, PhD; Marszalski, Maciej MD; Piwoda, Maciej MD; Molsa, Maciej MD; Pietka, Marek MD; Filipiak, Kamil MD; Miechowicz, Izabela PhD; Czarnik, Tomasz MD, PhD1. Infraclavicular, Ultrasound-Guided Percutaneous Approach to the Axillary Artery for Arterial Catheter Placement: A Randomized Trial. Critical Care Medicine ():10.1097/CCM.0000000000006015, August 07, 2023. | DOI: 10.1097/CCM.0000000000006015
Category: Critical Care
Posted: 8/15/2023 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Background:
There has been interest in vitamin C as an adjunctive therapy in patients with systemic inflammation and vasoplegia to reduce inflammation. While it was suggested that vitamin C may have some benefit (along with hydrocortisone and thiamine) in septic shock, the LOVIT trial showed possible harm from high-dose vitamin C administration in septic ICU patients. The VALENCIA trial sought to evaluate whether vitamin C could reduce the duration of vasopressor therapy in patients with moderate vasoplegic shock.
Study:
-double-blinded RCT at two tertiary centers, 71 patients (36 to placebo, 35 to vitamin C)
-adult patients with vasoplegic shock of any cause
-vasopressor requirement >10 μg/min of norepi after hypovolemia was excluded
-notable exclusion criteria: end-stage renal failure and expected survival <12 hrs
Results:
-65 pts with septic shock, 6 pts with non-infectious cause
-no significant difference in the duration of vasopressors between the treatment group (median, 44 h [95% CI, 37-54 hrs]) and the control group (55 hrs [95% CI, 33-66 hrs])
-also no statistically significant difference in the vasopressor dose at 12 hourly time points, ICU or 28-day mortality and ICU or hospital length of stay
Take-home points:
Small study that ultimately may be under-powered but did not show that vitamin C reduces vasopressor duration in moderate vasoplegic shock
Anstey MH, Aljeaidi MS, Palmer R, et al. Intravenous vitamin C for vasoplegia: A double-blinded randomised clinical trial (VALENCIA trial). Journal of Critical Care. 2023;78:154369.
Category: Critical Care
Posted: 8/8/2023 by William Teeter, MD
Click here to contact William Teeter, MD
Targeted Therapeutic Mild Hypercapnia after Resuscitated Cardiac Arrest (TAME)
Current guidelines recommend normocapnia for out-of-hospital cardiac arrest (OHCA), the TAME Study asked is mild hypercapnia better?
Conclusion: "In patients with coma who were resuscitated after out-of-hospital cardiac arrest, targeted mild hypercapnia did not lead to better neurologic outcomes at 6 months than targeted normocapnia."
https://www.nejm.org/doi/full/10.1056/NEJMoa2214552
https://clinicaltrials.gov/study/NCT03114033
https://www.thebottomline.org.uk/blog/ebm/tame/
Category: Critical Care
Keywords: OHCA, ROSC, cardiac arrest, resuscitation, CT, pan-scan, computed tomography (PubMed Search)
Posted: 7/25/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
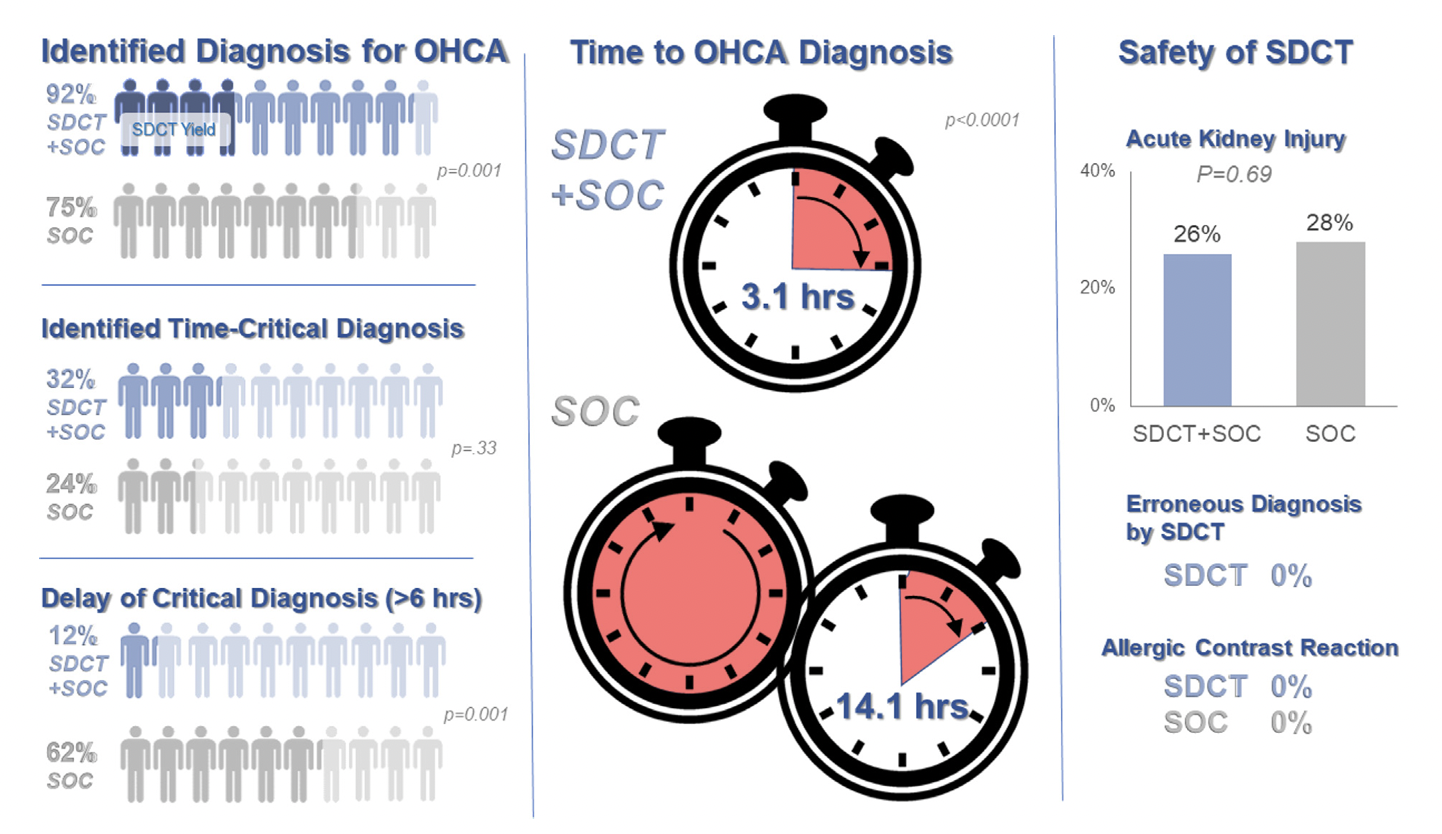
Background: Prior evidence1,2 has suggested that early “pan-scan” after ROSC provides clinically-relevant information that assists in the care of the patient in question, when the cause of OHCA is unclear.
The recent CT FIRST trial looked at patients pre- and post- implementation of a protocol for head-to-pelvis CT within 6 hours of ROSC for adult patients without known cause or evidence of possible cardiac etiology, stable enough for scan. *Patients with GFR <30 were excluded from assignment to CT, although were included in the post/CT cohort if their treating doctors ordered CT scans based on perceived clinical need. To balance this, a similar number of patients with GFR <30 were included in the pre/“standard of care” cohort.
Outcomes After Protocol (Pre- vs. Post-):
Bottom Line: Early pan-CT allows for earlier definitive diagnosis and stabilization without increase in adverse events. While this earlier diagnosis does not seem to yield better survival, earlier stabilization may provide some benefits in terms of resource allocation and disposition, a notable benefit during our current crisis of staffing shortages and ED boarding.
Category: Critical Care
Keywords: Central Lines, Platelets, Bleeding (PubMed Search)
Posted: 7/18/2023 by Mark Sutherland, MD
(Updated: 2/9/2026)
Click here to contact Mark Sutherland, MD
Central Venous Catheter (CVC; aka central line) placement is a common procedure in both the ED and ICU, and while overall quite safe, does carry some risk. In particular, many of us regularly are confronted with the challenge of placing a line in a patient with profound thrombocytopenia, which can result in significant bleeding. In these cases, should we give platelets before we place the line?
Van Baarle et al published a randomized study in NEJM comparing an empiric 1u platelet transfusion vs no transfusion in patients with a platelet count of 10,000-50,000, prior to line placement. The study included both HD and non-HD (e.g. TLC) lines, from all three major access sites, in patients in their ICU or hematology ward. They found statistically fewer serious bleeding events in the transfusion group (4.8%) vs no transfusion group (11.9%). The study wasn't powered to look at more patient oriented outcomes like mortality, but I'm sure we can all agree less bleeding is probably a good thing. Also importantly, this study did not evaluate the risks/benefits of delaying line placement to obtain platelets when the line is urgently needed, so I would not recommend extending this to conclude platelets must be given before line placement if the line is needed for something highly time-sensitive (e.g. only available access to infuse pressors in a hypotensive patient).
Bottom Line: It is probably beneficial and appropriate to provide prophylactic platelet transfusion prior to CVC placement in patients with a platelet count less than 50,000, assuming circumstances allow.
van Baarle FLF, van de Weerdt EK, van der Velden WJFM, Ruiterkamp RA, Tuinman PR, Ypma PF, van den Bergh WM, Demandt AMP, Kerver ED, Jansen AJG, Westerweel PE, Arbous SM, Determann RM, van Mook WNKA, Koeman M, Mäkelburg ABU, van Lienden KP, Binnekade JM, Biemond BJ, Vlaar APJ. Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia. N Engl J Med. 2023 May 25;388(21):1956-1965. doi: 10.1056/NEJMoa2214322. PMID: 37224197.
https://www.nejm.org/doi/10.1056/NEJMoa2214322
Category: Critical Care
Keywords: NEWS, MEWS, IEWS, international Early Warning Score, mortality (PubMed Search)
Posted: 6/27/2023 by Quincy Tran, MD, PhD
(Updated: 2/9/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: Retrospective data from 3 Dutch EDs (development of the score), 2 Denmark ED (for validation of the score). The novel score (International Early Warning Score) will be composed of the National Early Warning Score (NEWS) + Age +Sex
Components of the National Early Warning Score:
Participants: All adult patients in the Netherlands Emergency department Evaluation Database (NEED) and Danish Multicenter Cohort (DMC).
Outcome measurement: in-hospital mortality, including death in EDs.
Study Results:
Discussion:
Conclusion:
This multicenter study showed that IEWS perform better than the NEWS for predicting in-hospital mortality for ED patients.
Candel BGJ, Nissen SK, Nickel CH, Raven W, Thijssen W, Gaakeer MI, Lassen AT, Brabrand M, Steyerberg EW, de Jonge E, de Groot B. Development and External Validation of the International Early Warning Score for Improved Age- and Sex-Adjusted In-Hospital Mortality Prediction in the Emergency Department. Crit Care Med. 2023 Jul 1;51(7):881-891. doi: 10.1097/CCM.0000000000005842. Epub 2023 Mar 23. PMID: 36951452; PMCID: PMC10262984.
