Category: Critical Care
Keywords: OCHA, VF, ventricular fibrillation, cardiac arrest, shockable, Occult VF (PubMed Search)
Posted: 1/28/2026 by Kami Windsor, MD
(Updated: 2/8/2026)
Click here to contact Kami Windsor, MD
A crucial part of cardiac arrest management is identification of the underlying rhythm, with key aspects of management diverging depending whether shockable (pulseless ventricular tachycardia/pVT or ventricular fibrillation/VF) or unshockable (pulseless electrical activity/PEA or asystole).
A recent study prospectively evaluated adult atraumatic out-of-hospital-cardiac-arrests (OHCAs) presenting to the ED, to determine what percentage of cases had “Occult VF” – VF found point-of-care echocardiogram but not by ECG. The researchers only included cases with simultaneous ECG and echo assessments for the initial 3 pulse checks. Echo and ECG determinations for the study were adjudicated by research team members.
They found that:
Major limitations:
Bottom Line: Point-of-care echocardiogram continues to have value in the management of cardiac arrest, potentially changing management and affecting post-ROSC decisions. Ensuring high-quality CPR, with appropriate defibrillation and anti-arrhythmic strategies, remains paramount in management of shockable OHCA.
Gaspari R, Adhikari S, Gleeson T, et al. Occult Ventricular Fibrillation Visualized by Echocardiogram During Cardiac Arrest: A Retrospective Observational Study From the Real-Time Evaluation and Assessment for Sonography-Outcomes Network (REASON). J Am Coll Emerg Physicians Open. 2025;6(1):100028. doi: 10.1016/j.acepjo.2024.100028.
Category: Critical Care
Keywords: Shock, procedures, arterial line, blood pressure, mean arterial pressure, MAP (PubMed Search)
Posted: 12/2/2025 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
We have all been there – an ED patient with circulatory shock requiring vasoactive medications and, therefore, an arterial line for accurate and close monitoring of the MAP and appropriate titration of the infusions. But does it save lives?
The recently published NEJM article by Muller et al. takes a look at noninvasive BP monitoring (NIBP) by cuff versus early arterial catheterization in patients with hypotension and evidence of tissue hypoperfusion:
Bottom Line: This trial indicates that in appropriately-selected patients with shock, such as those not on high doses of vasopressors, with BMI < 40 and an ability to consistently obtain NIBP measurements, early arterial line placement in the ED for vasopressor titration is unlikely to improve outcomes. It is important to note other potential indications for arterial line placement (severe hypoxia, inability to obtain reliable SpO2 with need for ABG monitoring, cardiac arrest, pain related to NIBP cuff monitoring, intracranial hemorrhage, etcetera) may still make arterial line placement in the ED prudent and better for overall patient care.
*France refers to norepi by the tartrate formulation dose, US refers to the base norepi dose (ratio is 2:1 tartrate: base).
Muller G, Contou D, Ehrmann S, et al.; CRICS-TRIGGERSEP F-CRIN Network and the EVERDAC Trial Group. Deferring Arterial Catheterization in Critically Ill Patients with Shock. N Engl J Med. 2025;393(19):1875-1888. doi: 10.1056/NEJMoa2502136.
Category: Critical Care
Keywords: acute respiratory failure, hypercapnia, hypercarbia, COPD, AE-COPD, noninvasive ventilation, high flow nasal cannula (PubMed Search)
Posted: 10/7/2025 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Q: Can you use high flow nasal cannula (HFNC) to manage acute hypercapnic respiratory failure?
A: It probably depends.
Background: While we now frequently utilize HFNC as an initial therapy for most acute hypoxic respiratory failure, its appropriateness in managing acute respiratory failure with hypercarbia has historically been opposed. With more recent data indicating that HFNC may be as good as noninvasive ventilation (NIV) for management of hypercapnia as well, this seemed like a good time to point out a few things:
The RENOVATE trial was a larger multicenter randomized noninferiority trial looking at HFNC vs NIV in all-comer acute respiratory failure, summarizing that HFNC was noninferior in the primary composite outcome of death + intubation at 7 days.
BUT this conclusion is not clearly supported in the smaller COPD (or acute cardiogenic pulmonary edema) subgroup:
What does seem to be clear across studies that HFNC has the capacity to clear some CO2 and is by and large better tolerated than facemask NIV.
Bottom Line: For mild-moderate acute COPD exacerbations with patient intolerance or exclusion criteria for NIV therapy, trialing HFNC is a reasonable option. For patients with severe acute or acute on chronic hypercapnia, as indicated by a [pseudo-arbitrary] pH < 7.25 and PaCO2 >70-80, noninvasive ventilation should be your go-to… or be ready to promptly intubate if/when the high flow fails.
Category: Critical Care
Keywords: intubation, sedation, rapid sequence intubation, RSI, rocuronium, succinylcholine, etomidate, ketamine, propofol (PubMed Search)
Posted: 8/12/2025 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Whether you agree or disagree that “roc rocks and succ sucks,” evidence shows that approximately 3-4% of intubated patients experience awareness while paralyzed [1,2], and more of these patients are in the rocuronium subgroup [2,3,4]. Rocuronium acts in a dose-dependent fashion; the relatively standard 1-1.2 mg/kg in emergency department rapid sequence intubation (RSI) can result in a duration of paralysis can of up to 60-90 minutes. Commonly used sedatives in RSI, however, such as etomidate and ketamine, wear off quickly, before before rocuronium's paralytic effects have abated.
A recent single-center study showed that the majority of patients (60%) receiving rocuronium for paralysis during rapid sequence intubation (RSI) received no additional sedation until more than 15 minutes after induction, whether in the ED or ICU [5].
Patients experiencing awareness during paralysis with post-traumatic stress disorder [1,2] including distress from being restrained, feeling procedures, and feeling of impending death.
Bottom line: Start appropriate dose sedation promptly after RSI, especially with rocuronium, to avoid short- and long-term distress to patients.
Category: Critical Care
Keywords: OHCA, shockable rhythms, VF, ventricular fibrillation, defibrillation, AED, energy (PubMed Search)
Posted: 6/4/2025 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
A recent retrospective cohort study out of China investigated an escalating energy (200 > 300 > 360J) versus fixed energy (200 > 200 > 200 J) defibrillation strategy in OHCA with ventricular fibrillation requiring repeated defibrillations.
Notes:
Results:
Caveats:
Category: Critical Care
Keywords: OHCA, cardiac arrest, refractory VT/VF, shockable, ventricular arrhythmia, amiodarone, lidocaine (PubMed Search)
Posted: 4/2/2025 by Kami Windsor, MD
(Updated: 2/8/2026)
Click here to contact Kami Windsor, MD
A 2023 retrospective cohort study comparing amiodarone to lidocaine for in-hospital cardiac arrests (IHCA) with refractory VT/VF found that use of lidocaine was associated with increased chance of ROSC, 24 hour survival, survival to discharge, and favorable neurologic outcome at hospital discharge.[1]
Now, a recent study comparing amiodarone to lidocaine in the pre-hospital setting for OHCA has found similar results. [2] Another retrospective cohort study using propensity score matching, they evaluated 23,263 adult patients with OHCA and defibrillation refractory VT/VF managed by 1700 EMS agencies.
Use of lidocaine was associated with greater odds of prehospital ROSC, fewer post-drug administration defibrillations, and greater odds of survival to discharge.
In comparison to earlier trials, these studies are some of the first demonstrating benefits to lidocaine use over amiodarone that reach statistical significance, but of course have all the limitations that come with retrospective studies and are not further analyzed in the context of etiologies for cardiac arrest or application of post-ROSC care.
Bottom Line: If you happen to be someone who reaches for amiodarone as your go-to, it may be time to start considering lidocaine.
Category: Critical Care
Keywords: OHCA, cardiac arrest, ROSC, post-arrest syndrome, post-arrest care (PubMed Search)
Posted: 2/5/2025 by Kami Windsor, MD
(Updated: 2/8/2026)
Click here to contact Kami Windsor, MD
For those of us living in a world where ED boarding is a reality and ICU beds are in short supply, a re-up on the basic tenets of post-arrest care to optimize survival and neurologic outcomes in patients with sustained ROSC after OHCA:
Category: Critical Care
Keywords: OHCA, cardiac arrest, IV, intravenous, IO, intraosseous, epinephrine (PubMed Search)
Posted: 1/29/2025 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Two recent studies (see “Additional Information” for more study details) published in the New England Journal of Medicine evaluated the outcomes of OHCA, comparing drug administration via intraosseous devices versus intravenous access, neither demonstrating benefit to one strategy over the other in terms of sustained ROSC or 30-day survival. [1,2] While there were a few limitations, these results are generally in line with existing literature. Although it is worth noting that some studies signal improved outcomes with IV access, the time to intervention seems to be the more important metric related to outcome. [3-5]
Bottom Line: Intraosseous devices remain rapid and easy to place devices that can provide access for drug administration when IV access is unable to be obtained. In patients with difficult access, use an IO to administer meds, fluids, or blood products as indicated while you and your team work on more definitive IV access and focus on high-quality CPR.
Couper et al.
Vallentin et al.
Category: Critical Care
Keywords: OHCA, opioid, opiates, fentanyl, overdose, cardiac arrest (PubMed Search)
Posted: 9/2/2024 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The incidence of opioid-overdose-related deaths has clearly increased in the past decade, with recent estimates of up to 17% of OHCA being opioid-related in 2023. [1,2] The use of naloxone for opiate reversal in overdose is well-established, with reasonable inference but no formal proof that its use could help in opioid-associated out of hospital cardiac arrest (OA-OHCA). [3] The August publication of two trials [4,5] retrospectively examining naloxone administration in OHCA offers some perspectives…
and
[View “Visual Diagnosis” for slightly more detail on the referenced studies.]
Bottom Line: While prospective trials are absolutely needed to offer more definitive evidence regarding the use of empiric naloxone in nontraumatic OHCA, the rising incidence of OA-OHCA in the U.S. and current findings are convincing enough to encourage early naloxone administration, especially in populations with higher incidence of opioid use.
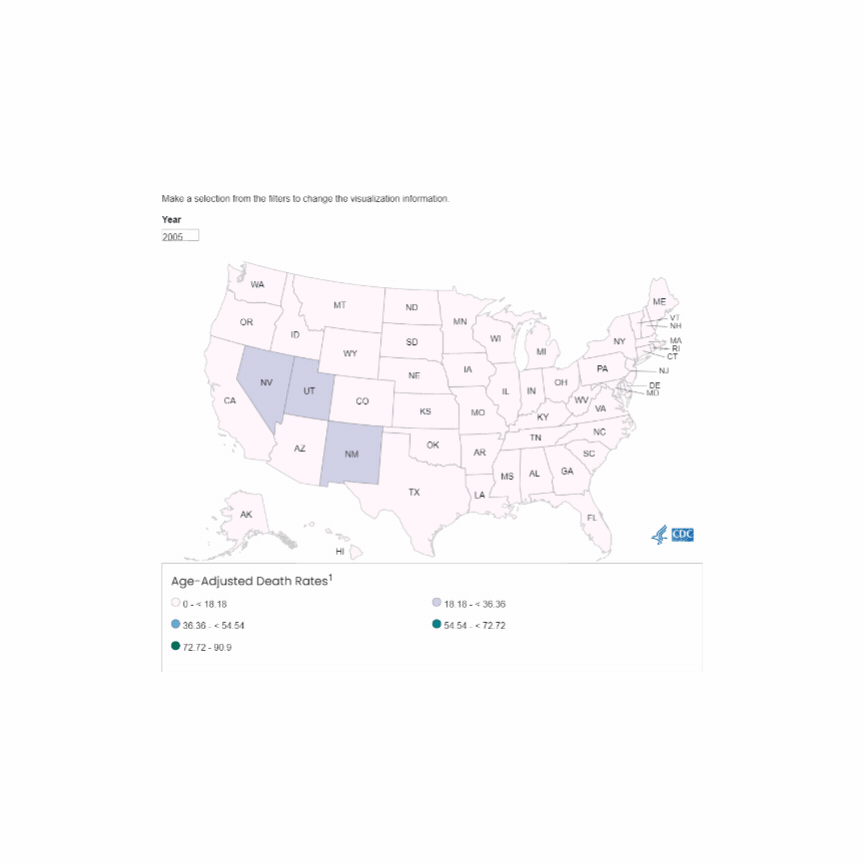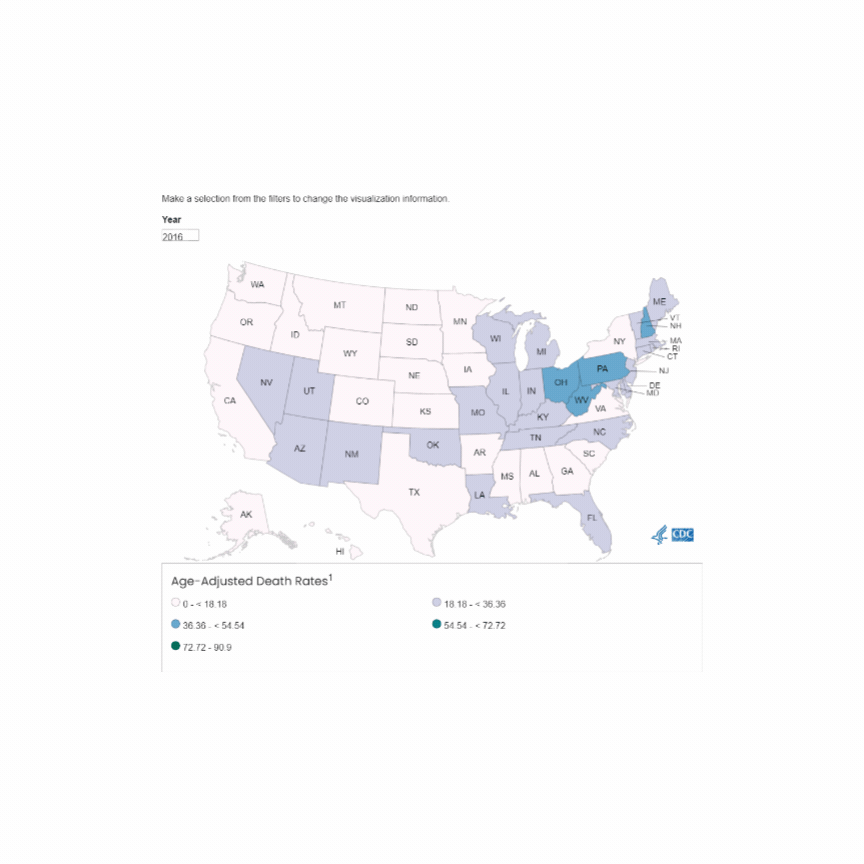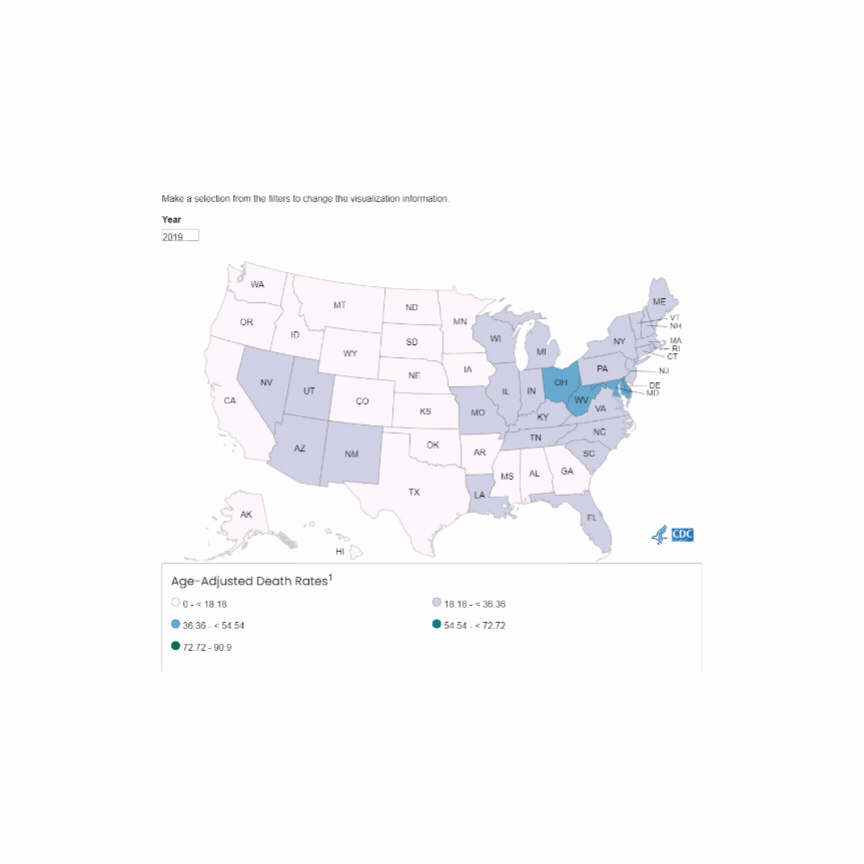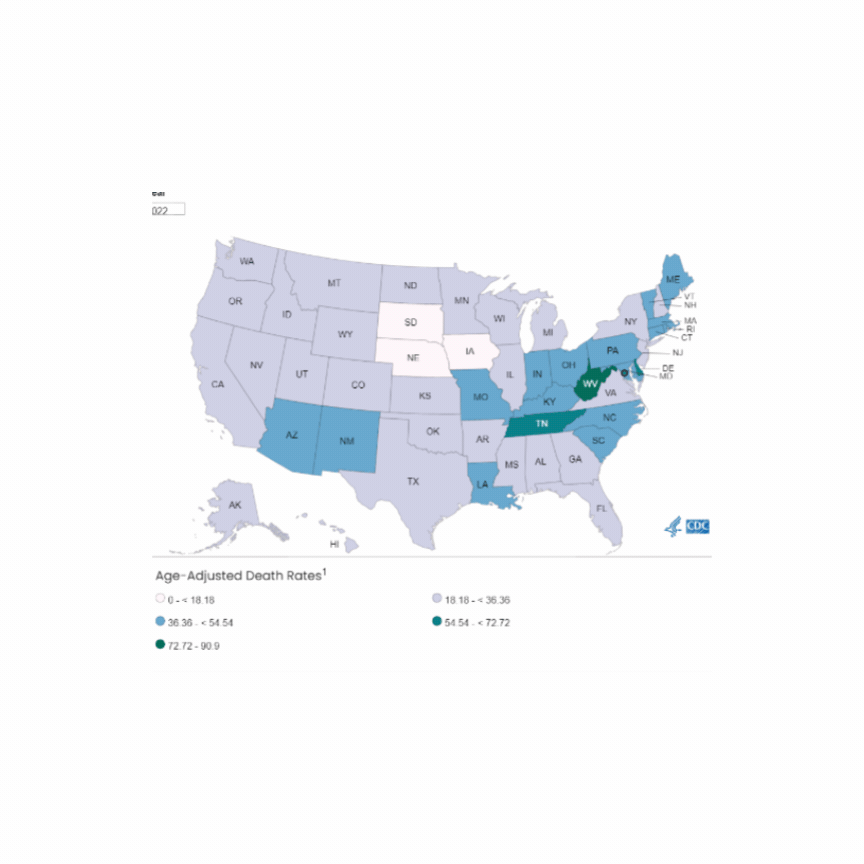
U.S. Mortality due to Opioid Overdose (CDC data)
Dillon et al, JAMA
Strong et al, Resuscitation
Category: Critical Care
Keywords: Corticosteroids, septic shock, ARDS, acute respiratory distress syndrome, community acquired pneumonia, CAP, dexamethasone, methylprednisolone, hydrocortisone (PubMed Search)
Posted: 7/9/2024 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
This May, the Society of Critical Care Medicine (SCCM) published new recommendations [1] for the use of corticosteroids in critical illness (separate from patients with known adrenal insufficiency or on chronic steroids), namely:
Bottom Line:
For severe bacterial pneumonia and septic shock, ED physicians should feel comfortable administering a dose of hydrocortisone 50mg IV as hydrocortisone 200mg/day is an accepted regimen for these disease processes.
For patients with ARDS who remain boarding in the ED, EM docs should discuss initiation of steroids with their intensivists, whether the institutional preference is for dexamethasone 20mg IV (per DEXA-ARDS) [6] or methylprednisolone 1mg/kg/day (per Meduri)[7].
Category: Critical Care
Keywords: sepsis, septic shock, warning scores (PubMed Search)
Posted: 6/25/2024 by Kami Windsor, MD
(Updated: 2/8/2026)
Click here to contact Kami Windsor, MD
Background: Sepsis remains a common entity associated with a relatively high rate of inpatient mortality, with timely recognition and treatment being key to improving patient outcomes. Various screening and warning scores have been created to attempt to identify sepsis and those patients at high risk of mortality earlier, but have limited performance because of suboptimal sensitivity and specificity.
A prospective observational study compared the performance of a variety of these scores (SIRS, qSOFA, SOFA, MEWS) as well as a machine learning model (MLM) against ED physician gestalt in diagnosing sepsis within the first 15 minutes of ED arrival.
Although not without its limitations, this study highlights the importance and relative accuracy of physician gestalt in recognizing sepsis, with implications for how to develop future screening tools and limit unnecessary exposure to unnecessary fluids and empiric broad spectrum antibiotics.
Bottom Line: In the era of machine learning models and AI, ED physicians are not obsolete. Even at 15 minutes, without lab results and diagnostics, our assessments lead to appropriate diagnoses and care. In this new normal of prolonged wait times and ED boarding, ED triage and evaluation models that optimize early physician assessment are of the utmost importance.
Knack SKS, Scott N, Driver BE, Pet al. Early Physician Gestalt Versus Usual Screening Tools for the Prediction of Sepsis in Critically Ill Emergency Patients. Ann Emerg Med. 2024 :S0196-0644(24)00099-4. doi: 10.1016/j.annemergmed.2024.02.009.
Category: Critical Care
Keywords: cardiac arrest, OHCA, airway, mechanical ventilation, resuscitation, bag-valve mask, manual ventilation (PubMed Search)
Posted: 4/10/2024 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
In cardiac arrest, avoidance of excessive ventilation is key to achieving HQ-CPR and minimizing decreases in venous return to the heart. The controversy regarding BVM vs definitive airway and OHCA outcomes continues, but data indicates that mechanical ventilation during CPR carries no more variability in airway peak pressures and tidal volume delivery than BVM ventilation [1], with the AHA suggestion to keep in-hospital cardiac arrest patients with COVID-19 on the ventilator during the pandemic [2].
So, can we automate this part of CPR?
Two recent studies looked at mechanical ventilation (MV) compared to bagged ventilation (BV) in intubated patients with out-of-hospital-cardiac arrest (OHCA).
Shin et al.'s pilot RCT evaluated 60 intubated patients, randomizing half to MV and half to BV, finding no difference in the primary outcome of ROSC or sustained ROSC, or ABG values, despite significantly lower tidal volumes and minute ventilation in the MV group [3].
Malinverni et al. retrospectively compared MV and BV OHCA patients from the Belgian Cardiac Arrest Registry, finding that MV was associated with increased ROSC although not with improved neurologic outcomes. Of note, patients across the airway spectrum were included (mask, supraglottic, intubated), and the mechanical ventilation was a bilevel pressure mode called Cardiopulmonary Ventilation (CPV) specific to their ventilators, specifically for use during cardiac arrest [4].
Bottom Line: Larger randomized trials will be necessary to get a definitive answer as to how mechanical ventilation affects outcomes in OHCA, but in instances where the cause of arrest is not primarily pulmonary (severe asthma, pneumothorax) and the ED is short-staffed or prolonged resuscitations are likely (such as in accidental hypothermic arrests), it is probably reasonable to keep patients on the ventilator:
Category: Critical Care
Keywords: ROSC, OHCA, cardiac arrest, shock, vasopressors, norepinephrine, noradrenaline, epinephrine, adrenalin (PubMed Search)
Posted: 3/19/2024 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Post-arrest shock is a common entity after ROSC. There is support for the use of continuous norepinephrine infusion over epinephrine to treat shock after ROSC, due to concerns about increased myocardial oxygen demand and associations with higher rates of rearrest [1,2] and mortality [2,3] with the use of epinephrine compared to norepinephrine, and increased refractory shock with use of epinephrine infusion after acute MI [4].
An article in this month’s AJEM compared norepinephrine and epinephrine infusions to treat shock in the first 6 hours post-ROSC in OHCA [5]. With a study population of 221 patients, they found no difference in the primary outcome of incidence of tachyarrhythmias, but did find that in-hospital mortality and rearrest rates were higher in the epinephrine group.
Bottom Line: Absent definitive evidence, norepinephrine should probably be the first pressor you reach for to manage post-arrest shock, especially if there is strong suspicion for acute myocardial infarction.
Category: Critical Care
Keywords: poisoning, intoxication, altered mental status, GCS, endotracheal intubation (PubMed Search)
Posted: 2/20/2024 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background: Acutely intoxicated / poisoned patients are commonly encountered in the ED, with the classic teaching that a GCS < 9 is an indication to intubate for airway protection. But we’ve probably all had a patient who was borderline, or who we thought was still protecting their airway pretty well despite a lower GCS. Are we risking our patient’s health and our careers by holding off on intubation? Maybe not.
The NICO trial, a multicenter, randomized controlled trial, looked at patients presenting by EMS with GCS <9 due to suspected poisoning, without immediate indication for intubation (defined by signs of respiratory distress with hypoxia, clinical suspicion of any brain injury, seizure, or shock with systolic BP <90 mmHg). They found that withholding intubation with close monitoring, compared to the standard practice of intubating at the EMS or ED physician’s discretion, resulted in:
Comparing the patients who were intubated in each group, there was no significant difference between groups in:
Notes:
Bottom Line: Without clear indication for intubation such as respiratory distress or accompanying head bleed, etcetera, intubation for mental status alone shouldn't be dogma in acute intoxication. Close monitoring will identify need for intubation, without apparent worsened outcomes due to a watchful waiting approach.
Freund Y, Viglino D, Cachanado M, et al. Effect of Noninvasive Airway Management of Comatose Patients With Acute Poisoning: A Randomized Clinical Trial. JAMA. 2023; 330(23):2267-2274. doi: 10.1001/jama.2023.24391.
Category: Critical Care
Keywords: sepsis, antibiotics, AKI, ACORN, zosyn, piperacillin-tazobactam, cefepime (PubMed Search)
Posted: 1/31/2024 by Kami Windsor, MD
(Updated: 2/8/2026)
Click here to contact Kami Windsor, MD
Background: For better or worse, the combination of “vanc-and-zosyn” has long been a go-to empiric regimen for the treatment of septic shock. Piperacillin-tazobactam is known to cause decreased creatinine secretion into the urine leading to an increased serum creatinine without any actual physiologic harm to the kidney, but the results of previous studies have led researchers to posit an increase in actual AKI with the vanc and zosyn combo. This concern has led to some physicians choosing cefepime for anti-pseudomonal gram-negative coverage instead, despite its known potential for neurotoxicity and cefepime-associated encephalopathy.
The ACORN trial: The recently published ACORN trial compared cefepime to piperacillin-tazobactam in adult patients presenting to the ED or medical ICU with sepsis or suspected serious infection. The primary outcome was a composite of highest stage of AKI or death at 14 days.
Results:
Bottom Line: Good antibiotic stewardship would probably decrease the frequency of vanc-and-zosyn administration, but concern for renal dysfunction alone shouldn’t guide the choice between cefepime or piperacillin-tazobactam, even in those with CKD, and even in those patients also receiving vancomycin.
Qian ET, Casey JD, Wright A, et al. Cefepime vs Piperacillin-Tazobactam in Adults Hospitalized With Acute Infection: The ACORN Randomized Clinical Trial. JAMA. 2023 Oct 24;330(16):1557-1567. doi: 10.1001/jama.2023.20583.
Category: Critical Care
Keywords: BRASH, shock, av nodal blockers (PubMed Search)
Posted: 9/20/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The BRASH syndrome (Bradycardia, Renal failure, AV nodal blockade, Shock, Hyperkalemia) has been increasingly described in the literature in the past 3-5 years.
The inciting factor is generally considered to be something that prompts acute kidney injury, often hypovolemia of some sort. Rather than AV nodal blocker overdose or severe hyperkalemia causing conduction problems, the combination of AV nodal blocker use (most often beta-blockers, but can be any type) and hyperkalemia (often only moderate) has a synergistic effect on cardiac conduction with ensuing bradycardia that can devolve into a cycle of worsening renal perfusion and shock.
Treatment is supportive, but most effective when the syndrome is recognized and all parts simultaneously managed. ED physicians should be familiar with its existence for targeted whole-syndrome stabilization and to avoid diagnostic delay.
Category: Critical Care
Keywords: OHCA, ROSC, cardiac arrest, resuscitation, CT, pan-scan, computed tomography (PubMed Search)
Posted: 7/25/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
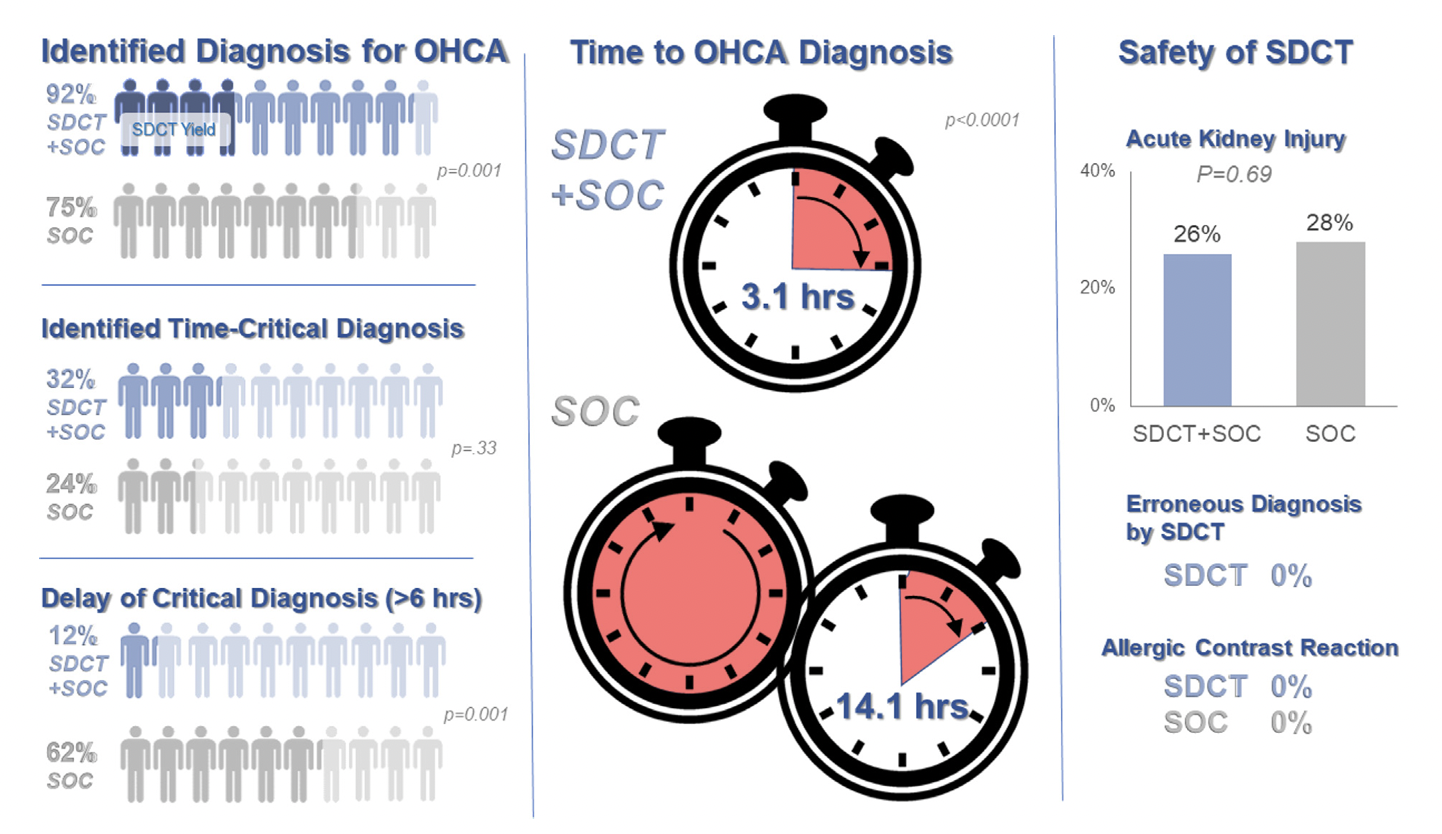
Background: Prior evidence1,2 has suggested that early “pan-scan” after ROSC provides clinically-relevant information that assists in the care of the patient in question, when the cause of OHCA is unclear.
The recent CT FIRST trial looked at patients pre- and post- implementation of a protocol for head-to-pelvis CT within 6 hours of ROSC for adult patients without known cause or evidence of possible cardiac etiology, stable enough for scan. *Patients with GFR <30 were excluded from assignment to CT, although were included in the post/CT cohort if their treating doctors ordered CT scans based on perceived clinical need. To balance this, a similar number of patients with GFR <30 were included in the pre/“standard of care” cohort.
Outcomes After Protocol (Pre- vs. Post-):
Bottom Line: Early pan-CT allows for earlier definitive diagnosis and stabilization without increase in adverse events. While this earlier diagnosis does not seem to yield better survival, earlier stabilization may provide some benefits in terms of resource allocation and disposition, a notable benefit during our current crisis of staffing shortages and ED boarding.
Category: Critical Care
Keywords: thrombocytopenia, bleeding, hemorrhage, platelets, transfusions, central lines, CVCs (PubMed Search)
Posted: 5/30/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background: In general practice, platelets are typically transfused for invasive procedures when the platelet count falls below 50 x 109/L. Regarding the placement of central venous catheters (CVCs), there is minimal data to support or refute decisions to transfuse platelets in these patients, although the 2015 Clinical Practice Guideline from the AABB (formerly, the American Association of Blood Banks) recommends deferring platelet transfusion until a platelet count of 20 x 109/L for CVC placement [weak recommendation, low quality evidence].1
In a study published this month in NEJM,2 van Baarle et al. performed a multicenter randomized controlled noninferiority trial comparing platelet transfusion to no transfusion in patients with platelets 10 to 50 x 109/L prior to US-guided CVC insertion. The primary outcome was the occurrence of catheter-related bleeding Grades 2-4 (Grade 1 = oozing; managed with <20 min of manual compression, not requiring RBC transfusion, & Grades 2-4 is everything else up to death) within 24 hours post-procedure.
Bottom Line: The jury is still out on best platelet transfusion practices prior to CVC placement, but I would strongly consider prophylactic platelet transfusion in patients with platelets < 30 x 109/L, those with underlying hematologic malignancy, and patients receiving larger CVCs such as dialysis lines. How much to transfuse in those with more severe thrombocytopenia is uncertain.
Separately, I would also strongly recommend use of US-guidance for any CVC placement in this population as well, based on practical common sense and some supportive literature as well.5
Additional Background: Data in pediatric oncology patients indicates that CVC placement with platelets <50 x 109/L is associated w/ increased occurence of minor but not major post-procedure bleeding,3 while adult data indicates that CVC placement can be performed until a threshold of 20 x 109/L before transfusions are needed to prevent severe bleeding.4
Additional Study Data:
Category: Critical Care
Keywords: pneumonia, acute hypoxic respiratory failure, steroids (PubMed Search)
Posted: 4/5/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background: The use of steroids in pneumonia has long been controversial with conflicting data, and the recent ESCAPe randomized controlled trial by Meduri et al. showing no mortality benefit with their use, but likely underpowered due to recruitment issues. The recently published CAPE COD study by Dequin et al. may change the game.
Design: Double-blind, placebo-controlled, multicenter, RCT
Intervention: Early hydrocortisone within 24 hrs, 200mg/day x 4-8 days depending on improvement, then preset taper
Primary outcome: Death at 28 days
Secondary outcomes:
Bottom Line: The addition of hydrocortisone to antibiotics in severe CAP may decrease need for intubation and development of shock, and in this well-done study, decreased 28 and 90-day mortality.
Meduri GU, Shih MC, Bridges L, et al; ESCAPe Study Group. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022 Aug;48(8):1009-1023. doi: 10.1007/s00134-022-06684-3. Epub 2022 May 13. PMID: 35723686.
doi: 10.1056/NEJMoa2215145. Epub ahead of print. PMID: 36942789.
Category: Critical Care
Keywords: sodium bicarbonate, bicarb, OHCA, cardiac arrest, CPR, resuscitation (PubMed Search)
Posted: 2/8/2023 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background: The use of sodium bicarbonate in the treatment of out-of-hospital cardiac arrest (OHCA) has been longstanding despite conflicting data regarding its benefit, outside of clear indications such as toxic ingestion or hyperkalemic arrest.
Study: A recent retrospective cross-sectional study by Niederberger et al.1 examined prehospital EHR data for ALS units responding to nonpregnant adults with nontraumatic OHCA, noting use of prehospital bicarb and the outcomes of 1) ROSC in the prehospital encounter and 2) survival to hospital discharge. They created propensity-matched pairs of bicarb and control patients, with a priori confounders: age, sex, race, witnessed status, bystander CPR, prearrival instructions, any defibrillation attempt, use of CPR feedback devices, any attempted ventilation, length of resuscitation, number of epi doses.
There were 23,567 arrests (67.4% asystole, 16.6% PEA, 15.1% VT/VF), 28.3% overall received sodium bicarb.
Results:
In the propensity-matched sample, survival was higher in bicarb group (5.3% vs. 4.3%; p=0.019).
There were no differences in rate of ROSC overall, but looking at the different rhythms, ROSC was higher in the bicarb group with asystole as the presenting rhythm (bicarb 10.6 vs 8.8%; p=0.013) but not PEA or VT/VF.
*There is no indication by the authors as to the dosing of bicarb most associated with survival to hospital discharge (or ROSC in asystole) in the study, however a previous study has indicated that a single amp of bicarb is unlikely to significantly improve severe metabolic acidosis (pH <7.1),2 so the general recommendation of at least 1-2mEq/kg should be employed.
Bottom Line: The use of sodium bicarb may increase survival in OHCA with initial PEA/asystole. The recommended initial dose is 1-2mEq/kg; giving at least 2 amps of bicarb (rather than the standard 1) should achieve this in many patients.
Between 1/2019 and 12/2020, there were 23,567 arrests that met inclusion criteria.
Overall EMS ROSC: 18.4%
Overall survival to hospital discharge: 7.6%
In the propensity-matched sample – survival was higher in bicarb group (5.3% vs. 4.3%; p=0.019).
There were no differences in rate of ROSC overall, but looking at the different rhythms, ROSC was higher in the bicarb group with asystole as the presenting rhythm (bicarb 10.6 vs 8.8%; p=0.013) but not PEA or VT/VF.
Overall, bicarb use was associated with improved survival (OR 1.25 (1.04-1.51) / aOR 1.3 (1.06-1.59) but not increased ROSC.
