Category: Pediatrics
Keywords: dehydration (PubMed Search)
Posted: 11/28/2014 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Dehydration is a common pediatric ED presentation. Oral rehydration (although first choice) is often not possible secondary to patient cooperation and/ or persistent vomiting. Intravenous (IV) hydration is often difficult, requiring multiple attempts especially in the young dehydrated infant.
Hyaluronan is a mucopolysaccharude present in connective tissue that prevents the spread of substances through the subcutneous space. Hyaluronidase is a human DNA-derived enzyme that breaks down hyaluronan and temporarily increases its permeability, thereby allowing fluid to be absorbed with the capillary and lymphatic systems.
In one study, patients age 1 month to 10 years were randomized to recieve 20 mL/kg bolus NS via subcutaneous (SC) or IV route over one hour, then as needed. The mean volume infused in the ED was 334.3 mL (SC) vs 299.6 mL (IV). Succesful line placement occured in all 73 SC patients and only 59/75 IV patients. There was a higher proportion of satisfaction for clinicians and parents for ease of use and satisfaction, respectively.
Bottom line: Consider subcutaneous hyaluronidase faciliated rehydration in mild to moderately dehydrated children, especially with difficult IV access.
Spandorfer PR, et al. A Randomized Clinical Trial of Recombinant Human Hyaluronidase-Fcilitated Subcutaneous Versus Intravenous Rehydration in Mild to Moderately Dehydrated Children in the Emergency Department. Clinical Therapeutics, 2012; 34(11): 2232-2245.
Category: Pediatrics
Keywords: E. coli, O0157:H7, hematochezia, diarrhea (PubMed Search)
Posted: 9/26/2014 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
There are numerous different causes of pediatric hemorrhagic diarrhea. Consider a pediatric patient with bloody diarrhea as being at risk for developing hemolytic uremic syndrome. Most cases of hemolytic uremic syndrome are caused by O157:H7 strains of E Coli that release Shiga-like toxin from the gut. Systemic release of the toxin causes microvascular thromboses in the renal microvasculature. The characteristic microangiopathic hemolysis results with anemia, thrombocytopenia and peripheral schistocytes seen on laboratory studies, in addition to acute renal failure.
Antibiotics have been controversial in the treatment of pediatric hemorrhagic diarrhea due to concern that they worsen toxin release from children infected with E Coli O157:H7 and thus increase the risk of developing hemolytic uremic syndrome. Numerous previous studies have provided conflicting data regarding the true risk (1). A recent prospective study showed antibiotic treatment increases the risk (2). Most recommendations warn against using antibiotics to treat pediatric hemorrhagic diarrhea unless the patient is septic.
Bottom line: Avoid treating pediatric hemorrhagic diarrhea with antibiotics
References:
1. Systematic review: are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection? Alimentary Pharmacology & Therapeutics. Volume 24, Issue 5, pages 731–742, September 2006
2. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin Infect Dis. 2012 Jul;55(1):33-41. doi: 10.1093/cid/cis299. Epub 2012 Mar 19.
Category: Pediatrics
Posted: 12/27/2013 by Mimi Lu, MD
(Updated: 12/28/2013)
Click here to contact Mimi Lu, MD
Category: Pediatrics
Keywords: trauma, cardiac arrest, return of spontaneous circulation (PubMed Search)
Posted: 11/22/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Category: Pediatrics
Keywords: sedation, pain management (PubMed Search)
Posted: 7/3/2013 by Mimi Lu, MD
(Updated: 7/26/2013)
Click here to contact Mimi Lu, MD
Cringing at the thought of sewing up another screaming 2 year old?
Consider intranasal fentanyl.
Who: Young, otherwise healthy pediatric patients undergoing minor procedures (laceration repair, fracture reduction/splinting, etc...)
What: Fentanyl (2mcg/kg)
When: 5 minutes pre-procedure
Where: Intranasal
Why: More effective than PO, less invasive than IV while being equally efficacious.
How: Use an atomizer, splitting the dose between each nostril.
Category: Pediatrics
Keywords: NIV, intubation (PubMed Search)
Posted: 6/28/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Category: Pediatrics
Posted: 4/26/2013 by Mimi Lu, MD
(Updated: 5/24/2013)
Click here to contact Mimi Lu, MD
Ultrasound findings of appendicitis
Ultrasound images:
http://www.youtube.com/watch?v=d9jKM6x52nk
http://sonocloud.org/watch_video.php?v=MWHM3D7KD25H
http://sonocloud.org/watch_video.php?v=54862AYWGHGA
Category: Pediatrics
Posted: 4/26/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
An overweight 5 year old male presents with acute onset abdominal pain that localizes to the right lower quadrant. What are some causes of a limited or nondiagnostic ultrasound study in children?
Acute appendicitis is a time sensitive diagnosis. Ultrasound is frequently used as the initial diagnostic imaging in children. There are several reasons why the appendix may not be visualized, including retro-cecal location, normal appendix, perforation, and inflammation around the distal tip. An additional clinical predictor associated with poor or inconclusive ultrasound results in appendicitis is increased BMI (body mass index).
A study examining 263 pediatric patients found when BMI > 85th percentile and clinical probability of appendicitis was <50%, 58% of ultrasounds were nondiagnostic. Children with a BMI <85th percentile and clinical probability of appendicitis was <50%, had nondiagonstic scans 42% of the time. These trends were also mimicked in the patients with a higher clinical probability of appendicitis. In the child with a nondiagnostic ultrasound, options include observation and repeat ultrasound scan or CT scan, both of which have associated risks.
Category: Pediatrics
Keywords: antibiotics, wait and see (PubMed Search)
Posted: 4/19/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
2013 AAP AOM Guidelines UPDATE
Category: Pediatrics
Posted: 3/29/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
You have diagnosed an infant or child with pneumonia. How do you decide if they need admission?
The Pediatric Infectious Disease Society and the British Thoracic Society each have guidelines from 2011 to help with this decision.
Category: Pediatrics
Posted: 3/23/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
In children, it is important to consider the maximum doses of local anesthetics when performing a laceration repair or painful procedure like abscess drainage. If there are multiple lacerations, or large lacerations, it may be possible to exceed those doses if one is not careful.
Max doses of common anesthetics
For example, in a 20 kg child (an average 5-6 year old), the maximum doses would be:
Pearls:
Category: Pediatrics
Posted: 2/22/2013 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Luu JL, Wendtland CL, Gross MF, et al. Three percent saline administration during pediatric critical care transport. Ped Emerg Care 2011;27(12):1113-1117
Category: Pediatrics
Keywords: magnets, bowel perforation, ischemic necrosis, ingestion (PubMed Search)
Posted: 11/30/2012 by Mimi Lu, MD
(Updated: 1/18/2013)
Click here to contact Mimi Lu, MD
Patient: A 10 year old female is brought to the ED after swallowing 2 beads (see image). Based on the findings, what are your concerns and what is the disposition?
Answer: Multiple Magnet Ingestion
The mother was eventually able to produce the magnetic beads ingested at home 2 hours prior to presentation
The ingestion of multiple magnets is a medical emergency. If the 2 magnets separate and reconnect it can lead to:
- pressure necrosis
- bowel perforation
- fistula formation
- and/or bowel obstruction secondary to kinking, inflammatory reaction, and/or internal herniation
Patients with a multiple magnet ingestion should be taken emergently to the OR for endoscopic evaluation.
If the magnets have passed the pylorus, conservative management with laxatives and serial X-rays may be performed, however if their position becomes fixed on serial imaging then an emergent laparotomy may need to be performed for the removal of the FBs before the symptoms and signs occur.
Bottom line: Patients presenting with a multiple magnet ingestion need to be admitted regardless of the FB location. Consult GI and pediatric surgery early, since prompt removal can prevent devastating outcomes. Single magnet ingestions can be managed conservatively with serial exams and imaging.
Reference:
Alzaham AM et al, Ingested magnets and gastrointestinal complications. Journal of Paediatrics and Child Health; 43 (2007) 497–498.
Category: Pediatrics
Posted: 12/28/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Category: Pediatrics
Posted: 12/15/2012 by Mimi Lu, MD
(Updated: 12/21/2012)
Click here to contact Mimi Lu, MD
Parents bring in their child who placed a bead, seed, or other object up her nose. What do you do? Who should you call?
Research suggests that a decades-old home remedy (of sorts) known as the “mother’s kiss” may do the trick for children 1-8 years of age. It’s also much less invasive or frightening than some of the tools and techniques used in emergency departments with a success rate approaching 60%
First described in 1965, here’s how the mother’s kiss technique works:
Category: Pediatrics
Keywords: meningitis, neck pain, retropharyngeal abscess (PubMed Search)
Posted: 11/16/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
A 1 year old gets sent from their pediatrician’s office for rule out meningitis. They presented with fever for 2 days and neck rigidity. Your LP results are normal. What additional test should you consider?
Answer:
Lateral neck x-ray
http://www.hawaii.edu/medicine/pediatrics/pemxray/v2c20.html
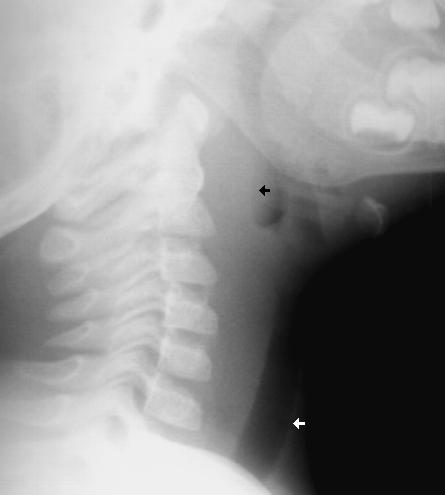
Retropharyngeal abscess (RPA) can commonly present like meningitis. Have a high suspicion in
children who are too young to complain of sore throat or difficulty swallowing.
A recent article in Pediatric Infectious Disease Journal detailed the rising incidence of retropharyngeal abscess, especially in younger patients, which is attributed to community acquired MRSA.
From 2004-2010 there was a 2.8 fold increase in RPA from the previous study period (1993-2003).
Children whose abscess grew MRSA were younger (mean 11 months) than the others (mean 62 months) (P < 0.001) and required longer duration of hospitalization (mean 8.8 days) than the rest (mean 4.5 days) (P = 0.002).
Bottom line: Consider a plain film in the child you are preparing to LP for meningitis.
Reference:
Abdel-Haq, N, Quezada M, Asmar BI. Retropharyngeal abscess in children: the rising incidence
of methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2012; 31: 696–699
Category: Pediatrics
Keywords: croup, laryngomalacia (PubMed Search)
Posted: 10/26/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Category: Pediatrics
Keywords: dysrhythmia, arrhythmia (PubMed Search)
Posted: 9/28/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
The incidence of pediatric syncope is common with 15%-25% of children and adolescents experiencing at least one episode of syncope before adulthood. Incidence peaks between the ages of 15 and 19 years for both sexes.
Although most causes of pediatric syncope are benign, an appropriate evaluation must be performed to exclude rare life-threatening disorders. In contrast to adults, vasodepressor syncope (also known as vasovagal) is the most frequent cause of pediatric syncope (61%–80%). Cardiac disorders only represent 2% to 6% of pediatric cases but account for 85% of sudden death in children and adolescent athletes. 17% of young athletes with sudden death have a history of syncope.
Key features on history and physical examination for identifying high-risk patients include exercise-related symptoms, a family history of sudden death, a history of cardiac disease, an abnormal cardiac examination, or an abnormal ECG.
Category: Pediatrics
Keywords: premedication, RSI, ventilator, high flow nasal cannula (PubMed Search)
Posted: 9/21/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
Category: Pediatrics
Keywords: septic shock, fluid resuscitation, PALS (PubMed Search)
Posted: 8/31/2012 by Mimi Lu, MD
Click here to contact Mimi Lu, MD
