Category: Critical Care
Keywords: posterior reversible encephalopathy syndrome, PRES, transplant, calcineurin inhibitors, tacrolimus, cyclosporine (PubMed Search)
Posted: 10/18/2022 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Emergency physicians are familiar with posterior reversible [leuko]encephalopathy syndrome as an entity associated with untreated hypertension. It also happens to be a well-documented entity amongst solid organ transplant patients.
While the exact pathophysiology remains unclear, PRES is characterized by posterior subcortical vasogenic edema due to blood-brain barrier disruption, usually in the setting of elevated blood pressure with loss of cerebral autoregulation and/or endothelial dysfunction.
The immunosuppressants used in this population, namely calcineurin inhibitors (CNI) such as tacrolimus and cyclosporine, are thought to contribute most to this endothelial dysfunction and development of PRES in transplant patients, although high-dose corticosteroids, ischemia-reperfusion injury during surgery, and antibiotics have also been implicated.
Presentation of PRES post-transplant:
Clinical symptoms:
Time course:
Diagnostics:
Management:
Bottom Line:
Patients with a history of solid organ transplant are at risk for PRES. While ED stabilization of these patients remains the same, recognition of PRES as a potential etiology for a transplant patient's presentation is crucial to proceed with important testing and necessary changes to their immunosuppressive regimen.
Category: Critical Care
Keywords: analgosedation, sedation, intubation, (PubMed Search)
Posted: 8/23/2022 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Deep sedation in the ED has previously been associated with longer duration of mechanical ventilation, longer lengths of stay, and higher mortality.1 Current guidelines recommend light sedation, consistent with a goal RASS of -2 to 0, for most critically-ill patients in the ICU.2
The ED-SED3 multicenter, pragmatic, before-and-after feasibility study implemented an educational initiative (inservices, regular reminders, laminated sedation charts) to help target lighter sedation depths in newly-intubated adult patients without acute neurologic injury or need for prolonged neuromuscular blockade.
After educational intervention:
Even with the caveats of the confounding and bias that can exist in before-and-after studies, these results are consistent with prior sedation-related studies and offer more evidence to support for avoiding deep sedation in our ED patients. The study also demonstrates the importance of nurse-driven sedation in achieving sedation goals.
Bottom Line: Our initial care in the ED matters beyond initial stabilization and compliance with measures and bundles. Avoid oversedating intubated ED patients, aiming for a goal RASS of -2 to 0.
Category: Critical Care
Keywords: Insulin infusion, diabetes mellitus, diabetic ketoacidosis, DKA, subcutaneous, long-acting (PubMed Search)
Posted: 6/29/2022 by Kami Windsor, MD
(Updated: 9/21/2022)
Click here to contact Kami Windsor, MD
Background: It is classically taught that the tenets of DKA management are IV fluids, electrolyte repletion, and an insulin infusion that is titrated until approximately 2 hours after anion gap closure, when long-acting subcutaneous insulin is administered if the patient is tolerating oral intake. It has been previously found that earlier administration of subcutaneous long-acting insulin can shorten the time to anion gap closure, while other small studies have noted similar efficacy in subcutaneous insulin compared to IV in mild/moderate DKA.
A recent JAMA article presents a retrospective evaluation of a prospectively-implemented DKA protocol (see "Full In-Depth" section) utilizing weight-based subcutaneous glargine and lispro, rather than IV regular insulin, as part of initial and ongoing floor-level inpatient treatment.
When compared to the period before the DKA protocol:
The only exclusion criteria were age <18 years, pregnancy, and presence of other condition that required ICU admission.
Bottom Line: Not all DKA requires IV insulin infusion.
At the very least, we should probably be utilizing early appropriate-dose subcutaneous long-acting insulin. With ongoing ICU bed shortages and the importance of decreasing unnecessary resource use and hospital costs, perhaps we should also be incorporating subcutaneous insulin protocols in our hospitals as well.
As a part of the DKA protocol, patients:
Elevated BMI was not included in exclusion criteria, however the authors note that their DKA protocol has been amended to exclude patients >166kg due to concerns regarding insulin absorption.
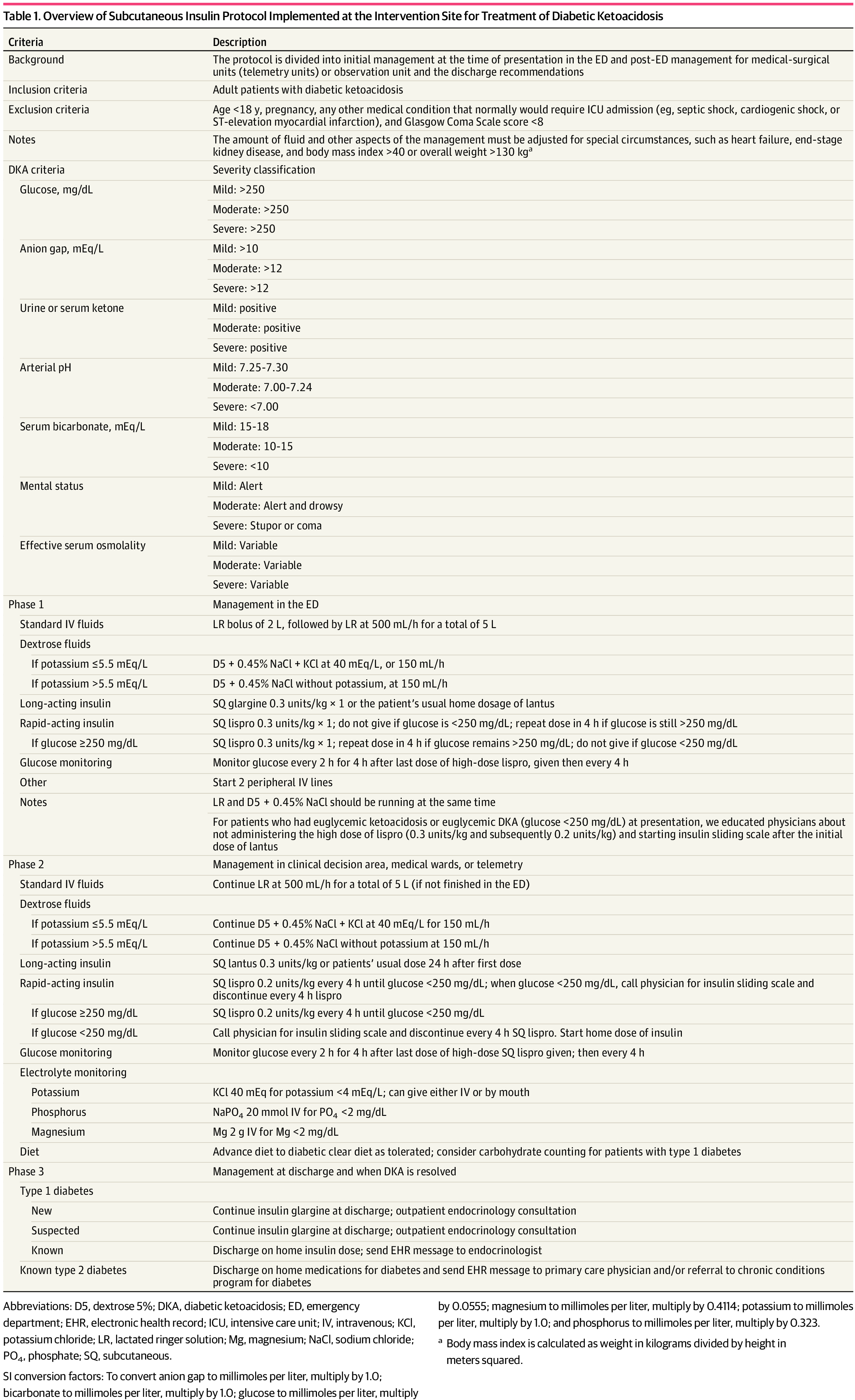
Rao P, Jiang S, Kipnis P, et al. Evaluation of Outcomes Following Hospital-Wide Implementation of a Subcutaneous Insulin Protocol for Diabetic Ketoacidosis. JAMA Netw Open. 2022;5(4):e226417. doi:10.1001/jamanetworkopen.2022.6417
Houshyar J, Bahrami A, Aliasgarzadeh A. Effectiveness of Insulin Glargine on Recovery of Patients with Diabetic Ketoacidosis: A Randomized Controlled Trial. J Clin Diagn Res. 2015 May;9(5):OC01-5. doi: 10.7860/JCDR/2015/12005.5883.
Mohamed A, Ploetz J, Hamarshi MS. Evaluation of Early Administration of Insulin Glargine in the Acute Management of Diabetic Ketoacidosis. Curr Diabetes Rev. 2021;17(8):e030221191986. doi: 10.2174/1573399817666210303095633.
Karoli R, Fatima J, Salman T, Sandhu S, Shankar R. Managing diabetic ketoacidosis in non-intensive care unit setting: Role of insulin analogs. Indian J Pharmacol. 2011 Jul;43(4):398-401. doi: 10.4103/0253-7613.83109.
Ersöz HO, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, Karti SS. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006 Apr;60(4):429-33. doi: 10.1111/j.1368-5031.2006.00786.x.
Category: Critical Care
Keywords: in-hospital cardiac arrest, IHCA, resuscitation, code, epinephrine, vasopressin, methylprednisolone (PubMed Search)
Posted: 5/2/2022 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Based on prior studies1 indicating possibly improved outcomes with vasopressin and steroids in IHCA (Vasopressin, Steroids, and Epi, Oh my! A new cocktail for cardiac arrest?), the VAM-IHCA trial2 compared the addition of both methylprednisolone and vasopressin to normal saline placebo, given with standard epinephrine resuscitation during in hospital cardiac arrest (IHCA).
The use of methylprednisolone plus vasopressin was associated with increased likelihood of ROSC: 42% intervention vs. 33% placebo, RR 1.3 (95% CI 1.03-1.63), risk difference 9.6% (95% CI 1.1-18.0%); p=0.03.
BUT there was no increased likelihood of favorable neurologic outcome (7.6% in both groups).
Recent publication on evaluation of long-term outcomes of the VAM-ICHA trial3 showed that, at 6-month and 1-year follow-up, there was no difference between groups in:
Bottom Line: Existing evidence does not currently support the use of methylprednisolone and vasopressin as routine code drugs for IHCA resuscitation.
Basic study characteristics:
Some of the limitations:
Category: Critical Care
Keywords: trauma, pneumothorax, positive pressure ventilation, invasive mechanical ventilation, tension pneumothorax (PubMed Search)
Posted: 1/14/2022 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background: Conventional medical wisdom long held that patients with pneumothorax (PTX) who require positive pressure ventilation (PPV) should undergo tube thoracostomy to prevent enlarging or tension pneumothorax, even if otherwise they would be managed expectantly.1
Bottom Line: The cardiopulmonar-ily stable patient with small PTX doesn’t need empiric tube thoracostomy simply because they’re receiving positive pressure ventilation. If you are unlucky enough to still have them in your ED at day 5 in these COVID times, provide closer monitoring as the observation failure rate may increase dramatically around this time.
Category: Critical Care
Keywords: OHCA, IHCA, targeted temperature management, therapeutic hypothermia, postcardiac arrest (PubMed Search)
Posted: 11/16/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Fever has long been understood to be associated with worse outcomes in patients post-cardiac arrest. Whether ascribing to the goal of 33-34°C, 36°C, or simply <38°C, close monitoring and management of core temperatures are a tenet of post-cardiac arrest care.
A recently published study compared the effectiveness of several methods in maintaining temperatures <38°C…
Results:
Maintenance of temp <38°C:
Mean change in temp from baseline:
Limitations:
Bottom Line:
Category: Critical Care
Keywords: IVF, intravenous fluids, resuscitation, infusion rates (PubMed Search)
Posted: 8/18/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background:
There are also no clear guidelines regarding how fast fluid boluses should be administered, and there has been debate about whether different infusion rates could lead to different outcomes in patients receiving intravenous fluid (IVF) boluses (i.e. fast infusions may cause more third spacing due to the rapidity of the expansion of the intravascular space compared to fluid administered more slowly). A recent study compared IVF infusion rates in ICU patients.
-- Unblinded, randomized
-- 10,520 patients clinically requiring a fluid challenge, from 75 ICUs in Brazil
-- Infusion rate 333 mL/hr vs 999 mL/hr
* (Trial also compared plasmalyte vs 0.9% saline, analyzed in separate study)
-- Some notable exclusion criteria: severe hypo/hypernatremia, AKI or expected to need RRT 6 hrs after admission
--Other caveats:
* Faster infusion rates allowed at physician discretion in patients with active bleeding or severe hypotension (SBP < 80 or MAP < 50 mmHg); patient was returned to assigned rate after condition resolved
* Almost 1/2 the patients received at least 1L of IVF in 24 hours prior to enrollment
-- Results: No sig difference in 90-day survival, use of RRT, AKI, mechanical ventilator free days, ICU/hospital mortality/LOS
Bottom Line: There is not yet compelling evidence that there are differences in patient outcomes in patients receiving fluid boluses given at 333 cc/hr vs. 999 cc/hr.
1. Zampieri FG, Machado FR, Biondi RS, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the basics randomized clinical trial. JAMA. Published online August 10, 2021.doi:10.1001/jama.2021.11444
2. Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0. 9% saline solution on mortality in critically ill patients: the basics randomized clinical trial. JAMA. Published online August 10, 2021.
Category: Critical Care
Keywords: cardiac arrest, IHCA, resuscitation, epinephrine, pediatrics (PubMed Search)
Posted: 8/11/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Approximately 15,000 children experience an in hospital cardiac arrest (IHCA) with little improvement in outcomes over the last two decades. During that time, epinephrine has been the constant basis for resuscitation of these patients. Current recommendations by the AHA recommend bolus dosing of epinephrine every 3-5 minutes in a pediatric cardiac arrest. Animal studies suggest that more frequent dosing of epinephrine may be beneficial.
This was a retrospective study of 125 pediatric IHCAs with 33 receiving “frequent epinephrine” interval (≤2 minutes). Pediatric CPC score 1-2 or no change from baseline was used as primary outcome to reflect favorable neurologic outcome, with frequent dosing associated with better outcome (aOR 2.56, 95%CI 1.07 to 6.14). Change in diastolic blood pressure was greater after the second dose of epinephrine among patients who received frequent epinephrine (median [IQR] 6.3 [4.1, 16.9] vs. 0.13 [-2.3, 1.9] mmHg, p=0.034).
This study is subject to all sorts of confounding and should be studied more rigorously, but suggests that more frequent dosing for pediatric IHCA may be of benefit.
Kienzle MF, Morgan RW, Faerber JA, et al. The Effect of Epinephrine Dosing Intervals on Outcomes from Pediatric In-Hospital Cardiac Arrest. Am J Respir Crit Care Med. 2021. doi: 10.1164/rccm.202012-4437OC.
Category: Critical Care
Keywords: cardiac arrest, CPR, cardiopulmonary resuscitation, hands-off time, CCF, chest compression fraction (PubMed Search)
Posted: 7/6/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Despite the knowledge that minimizing interruptions in chest compressions during CPR is key to maintaing coronary perfusion pressure and chance of ROSC,1-4 difficulties in limiting hands-off time remain.
Dewolf et al.5 recently performed a prospective observational study using body cameras to find that 33% (623/1867) of their CPR interruptions were longer than the recommended 10 seconds:
Previous studies have shown an increase in hands-off time associated with the use of cardiac POCUS during rhythm checks as well.6,7
Bottom Line:
Category: Critical Care
Keywords: cardiac arrest, ROSC, computed tomography, CT scan, imaging (PubMed Search)
Posted: 6/16/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
A recent prospective observational study examined the diagnostic usefulness of head-to-pelvis sudden death computed tomography (SDCT) in 104 patients with ROSC and unclear OHCA etiology.
Diagnostic performance:
Safety:
Bottom Line: For OHCA without clear etiology, SDCT explicitly including a thoracic CTA may have diagnostic benefit over standard care alone with the added benefit of identification of resuscitation complications.
Branch KRH, Strote J, Gunn M, et al. Early head-to-pelvis computed tomography in out-of-hospital circulatory arrest without obvious etiology. Acad Emerg Med. 2021 Apr;28(4):394-403. doi: 10.1111/acem.14228.
Category: Critical Care
Keywords: COPD, emphysema, acute respiratory failure, hypoxia, oxygen saturation (PubMed Search)
Posted: 4/20/2021 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Supplemental oxygen therapy is frequently required for patients presenting with acute respiratory distress and COPD exacerbation. Over-oxygenation can derail compensatory physiologic responses to hypoxia,1 resulting in worsening VQ mismatch and, to a lesser degree, decreases in minute ventilation, that cause worsened respiratory failure.
The 2012 DECAF (Dyspnea, Eosinopenia, Consolidation, Acidaemia, and Atrial Fibrillation) score was found to predict risk of in-hospital mortality in patients admitted with acute COPD exacerbation.2,3 Data from the DECAF study’s derivation and external validation cohorts were examined specifically to look at outcome associated with varying levels of oxygen saturation.
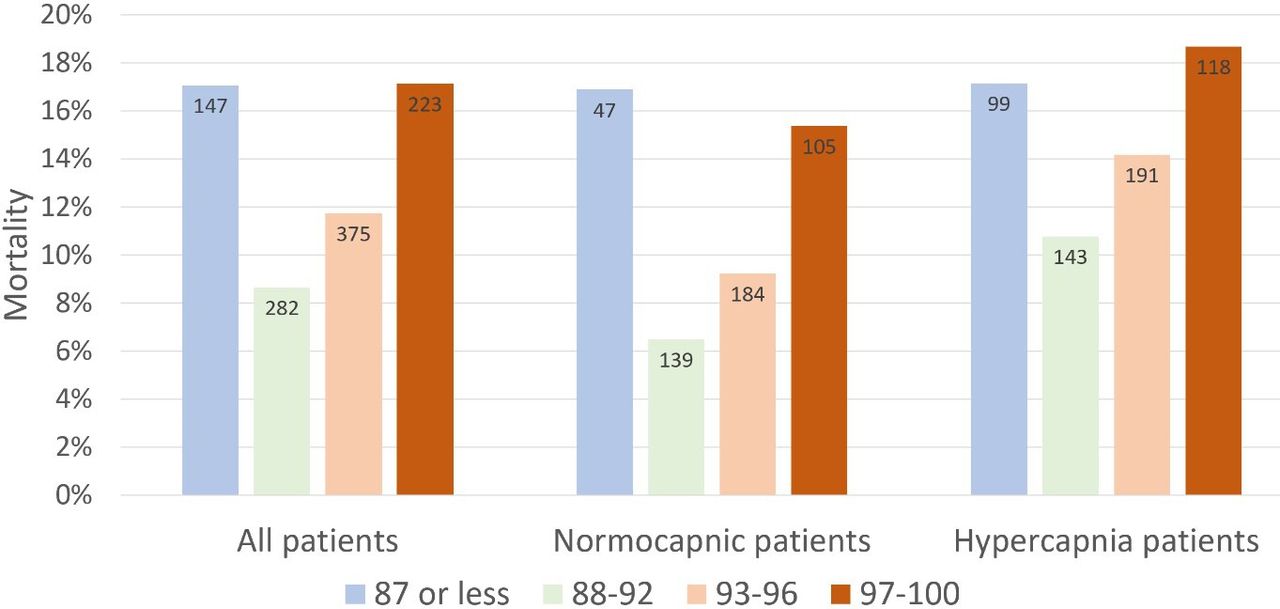
Bottom Line
In patients presenting to the ED with acute COPD exacerbation requiring oxygen supplementation, a target oxygen saturation of 88-92% is associated with the lowest in-hospital mortality, and higher oxygen saturations should be avoided independent of patients' PCO2 levels.
Category: Critical Care
Keywords: HACOR, NIV, noninvasive ventilation, acute respiratory failure (PubMed Search)
Posted: 2/2/2021 by Kami Windsor, MD
(Updated: 2/23/2021)
Click here to contact Kami Windsor, MD
Background: In respiratory failure due to COPD and cardiogenic pulmonary edema, noninvasive positive pressure ventilation decreases need for intubation and improves mortality,1 while its utility in other scenarios such as ARDS and pneumonia has yet to be proven.1,2 We know that patients on NIV with delays to needed intubation have a higher mortality,1,3 but intubation and mechanical ventilation come with risks that it is preferable to avoid if possible.
So how and when can we determine that NIV is not working?
The HACOR (Heart rate, Acidosis, Consciousness, Oxygenation, Respiratory rate) score at 1 hour after NIV initiation has been demonstrated to be highly predictive of NIV failure requiring intubation.4,5
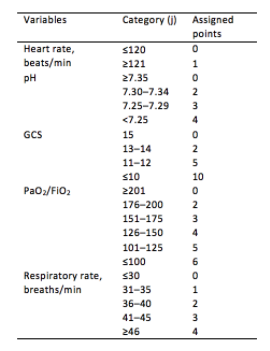
Initial development/validation: Score > 5 after 1 hour of NIV corresponds to >80% risk of NIV failure4
Earlier intubation (before 12 hours) in these patients = better survival
External validation: Score > 8 after 1 hour of NIV most predictive of eventual NIV failure 5
Average score @ 1-hour of patients with NIV success = 3.8
Score remained predictive at 6, 12, 24, 48 hours as well & mortality worsened as delay to intubation time increased
Baseline, pre-NIV score not predictive
Better predictive agreement in pneumonia and ARDS
Bottom Line:
Patients on NIV require close reassessment to prevent worsened survival due to intubation delay should invasive mechanical ventilation be indicated.
A HACOR score >8 after 1 hour of NIV should prompt intubation in most instances, with strong consideration given to a score >5.
*Note: ABGs were obtained for PaO2 assessment in the above studies -- the use of SpO2 was not evaluated -- but we are often not obtaining ABGs in our ED patients with acute respiratory failure. The following chart provides an estimated SpO2 to PaO2 conversion.
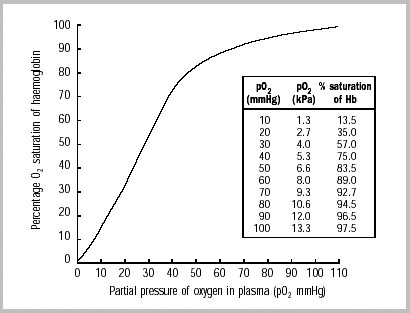
WHO 2001
Caveats:
Category: Critical Care
Keywords: airway management, cardiac arrest, COVID-10, SARS-CoV-2, cardiopulmonary resuscitation, CPR (PubMed Search)
Posted: 11/3/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
As the number of COVID-19 cases rises worldwide, prehospital and emergency department healthcare workers remain at high risk of exposure and infection during CPR for patients with cardiac arrest and potential SARS-CoV-2.
Existing evidence supports similar cardiac arrest outcomes in airways managed with a supraglottic airway (SGA) compared to endotracheal intubation (ETT).1 It is generally accepted that the best airway seal is provided with endotracheal intubation + viral filter, but how well do SGAs prevent spread of aerosols?
In CPR simulation studies:
Category: Critical Care
Keywords: resuscitation, ultrasound, VExUS, venous congestion (PubMed Search)
Posted: 9/8/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
While the invasive monitoring of central venous pressure (CVP) in the critically ill septic patient has gone the way of also transfusing them to a hemoglobin of 10 mg/dL, it remains that an elevated CVP is associated with higher mortality1,2 and renal failure.2,3
Extrapolating from existing data looking at hepatic vein, portal vein, and renal vein pulsatility as measures of systemic venous hypertension and congestion,4,5,6 Beaubien-Souligny et al. developed the venous excess ultrasound (VExUS) grading system incorporating assessment of all 3, plus the IVC, using US to stage severity of venous congestion in post-cardiac surgery patients.7 They evaluated several variations, determining that the VExUS-C grading system was most predictive of subsequent renal dysfunction.
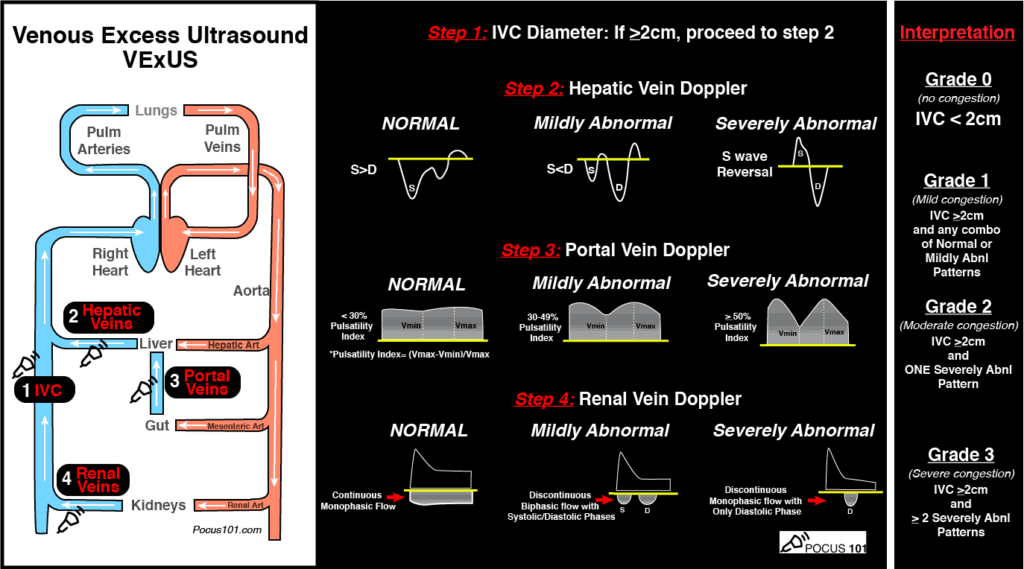
(Image from www.pocus101.com)
High Points
VExUS Grade 3 (severe) venous congestion:
Caveats
Clinical Uses
A great how-to can be found here:
https://www.pocus101.com/vexus-ultrasound-score-fluid-overload-and-venous-congestion-assessment/
1. Li DK, Wang XT, Liu DW. Association between elevated central venous pressure and outcomes in critically ill patients. Ann Intensive Care. 2017;7(1):83. doi:10.1186/s13613-017-0306-1
2. Chen KP, Cavender S, Lee J, et al. Peripheral Edema, Central Venous Pressure, and Risk of AKI in Critical Illness. Clin J Am Soc Nephrol. 2016;11(4):602-608.
3. Chen CY, Zhou Y, Wang P, Qi EY, Gu WJ. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: a meta-analysis. Crit Care. 2020;24(1):80. doi:10.1186/s13054-020-2770-5
4. Iida N, Seo Y, Sai S, et al. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4(8):674-682. doi:10.1016/j.jchf.2016.03.016
5. Ikeda Y, Ishii S, Yazaki M, et al. Portal congestion and intestinal edema in hospitalized patients with heart failure. Heart Vessels. 2018;33(7):740-751. doi:10.1007/s00380-018-1117-5.
6. Beaubien-Souligny W, Benkreira A, Robillard P, et al. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J Am Heart Assoc. 2018;7(19):e009961. doi:10.1161/JAHA.118.009961
7. Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12(1):16. doi:10.1186/s13089-020-00163-w
Category: Critical Care
Keywords: dexamethasone, steroids, respiratory failure, COVID-19, SARS-CoV-2, RECOVERY (PubMed Search)
Posted: 7/14/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The RECOVERY (Randomized Evaluation of COVid-19 thERapY) investigators recently published a non-peer reviewed article on their findings utilizing dexamethasone to treat patients with COVID-19.
Rx: Dexamethasone 6mg daily* x 10 days (PO or IV) *or steroid equivalent
Primary outcome: All-cause mortality at 28-days
Secondary outcomes:
Results:
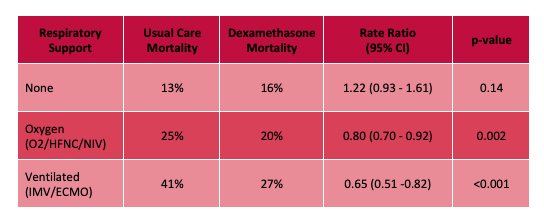
Limitations:
Bottom Line: Strongly consider admininstering dexamethasone to your patients with known COVID-19 who require respiratory support, and look for the peer-reviewed publication from the RECOVERY Trial investigators.
Horby P, Lim WS, Emberson et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1 (Accessed July 14th, 2020)
Category: Critical Care Literature Update
Keywords: sepsis, septic shock, acute renal failure, acute kidney injury, nephrotoxicity, vancomycin, MRSA, IV antibiotics (PubMed Search)
Posted: 5/27/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Background:
· Empiric broad spectrum antibiotic therapy is a mainstay of the management of critically ill patients with septic shock.
· Vancomycin is widely used for the coverage of potential MRSA infection
· Continuous infusion of vancomycin has been repeatedly demonstrated to reach target serum concentrations faster, maintain consistent serum vancomycin levels better, with fewer serum concentration sampling required, and less overall vancomycin required to do so, in both adult and pediatric populations.2-5
Current Article:
Flannery AH, Bissell BD, Bastin MT, et al. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: a Systematic Review and Meta-Analysis. Crit Care Med. 2020;48(6):912-8.
· Systematic review and meta-analysis of 11 studies for a total of 2123 patients
· Comparing continuous versus intermittent vancomycin infusion.
· Primary outcome of AKI, secondary outcome of mortality
· Found a reduction in the incidence of AKI in the continuous infusion cohort:
· No association between infusion strategy and mortality
Considerations:
· Initial loading dose used in most of the studies (15 mk/kg) probably underdosed, current recommendation for 25mg/kg initial loading dose7 (which is not even always effective by itself)8 (Reardon)
· Continuous infusion may be difficult with limited IV access
· AKI associated with increased hospital stay, costs, mortality (although didn’t pan out in study) – worth preventing if possible.
Take Home:
· Give a 25-30mk/kg loading dose of vancomycin in critically ill patients with suspicion of MRSA to achieve target serum concentrations sooner.
· Continuous vancomycin is a viable option and could be considered in ED boarders, especially if there is concern for impending renal injury.
1. Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin Plus Piperacillin-Tazobactam and Acute Kidney Injury in Adults: A Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(1):12?20. doi:10.1097/CCM.0000000000002769
2. Taheri M, Dadashzadeh S, Shokouhi S, Ebrahimzadeh K, Sadeghi M, Sahraei Z. Administration of Vancomycin at High Doses in Patients with Post Neurosurgical Meningitis: A Comprehensive Comparison between Continuous Infusion and Intermittent Infusion. Iran J Pharm Res. 2018;17(Suppl2):195?205.
3. Gwee A, Cranswick N, McMullan B, et al. Continuous Versus Intermittent Vancomycin Infusions in Infants: A Randomized Controlled Trial. Pediatrics. 2019;143(2):e20182179. doi:10.1542/peds.2018-2179
4. Vuagnat A, Stern R, Lotthe A, et al. High dose vancomycin for osteomyelitis: continuous vs. intermittent infusion. J Clin Pharm Ther. 2004;29(4):351?357. doi:10.1111/j.1365-2710.2004.00572.x
5. Hong LT, Goolsby TA, Sherman DS, et al. Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patients. J Crit Care. 2015;30(5):1153.e1?1153.e11536. doi:10.1016/j.jcrc.2015.06.012
6. Flannery AH, Bissell BD, Bastin MT, et al. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: a Systematic Review and Meta-Analysis. Crit Care Med. 2020;48(6):912-8.
7. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2020 Mar 19. doi: 10.1093/ajhp/zxaa036
8. Álvarez O, Plaza-Plaza JC, Ramirez M, et al. Pharmacokinetic Assessment of Vancomycin Loading Dose in Critically Ill Patients. Antimicrob Agents Chemother. 2017;61(8):e00280-17. doi: 10.1128/AAC.00280-17
Category: Critical Care
Keywords: Acute respiratory failure, respiratory distress, Coronavirus, COVID-19, SARS-CoV-2 (PubMed Search)
Posted: 4/11/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
There is currently a high, and appropriate, concern regarding the aerosolization of viral particles during various methods of respiratory support. While studies are limited, here is some of the currently available data (mostly-simulated) on the approximate maximum distances of particle spread:
Nasal Cannula 5LPM:1 1 ft 4.5 in
Non-Rebreather Mask, 6-12LPM: 4 in, minimal change with increasing flows1
High Flow Nasal Cannula
CPAP (20 cmH2O) provided by oronasal mask with good fit (leak from exhaust port):2 11.5 in
Bilevel positive airway pressure w/ oronasal mask (IPAP 10-18/EPAP 4): max dispersal:4 1 ft 7.7 in
Bilevel positive airway pressure with full facemask5 (IPAP 18 / EPAP 5): 2 ft 8 in
Bilevel positive airway pressure with helmet:4
Utility of Surgical Mask:6
Bottom Line:
In vivo data from actual patients is lacking, however there is potentially lower risk of aerosol spread with HFNC than regular nasal cannula, perhaps due to higher likelihood of a tighter nare/nasal cannula interface. Nonrebreather mask performs well indirectly with the shortest dispersal distance. Noninvasive positive pressure ventilation with an oronasal mask and good seal has a relatively short dispersal distance, and a surgical mask over respiratory support interventions actively decreases amount, if not distance, of particle spread. Use of appropriate PPE and negative pressure rooms, if available, remains key.
Category: Critical Care
Keywords: ACS, abdominal compartment syndrome, intraabdominal hypertension, emergent laparotomy (PubMed Search)
Posted: 2/18/2020 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
With ED-boarding of critically-ill patients becoming more common, it is likely that ED physicians may find themselves caring for a patient who develops ACS – that is, abdominal compartment syndrome. While intraabdominal hypertension (IAH) is common and is defined as intraabdominal pressure > 12 mmHg, ACS is defined as a sustained intraabdominal pressure > 20mmHg with associated organ injury.
WHY you need to know it:
ACS → Increased mortality & recognition is key to appropriate management
WHO is at risk:
HOW it kills:
→ Lactic acidosis, respiratory acidosis, multisystem organ failure, cardiovascular collapse & death
WHEN to consider it:
WHAT to do:
Bottom Line: Abdominal compartment syndrome is an affliction of the critically ill, is assosciated with worsened mortality, and requires aggressive measures to lower the intraabdominal pressure while obtaining emergent surgical consultation for potential emergent laparotomy.
Gottlieb M, Koyfman A, Long B. Evaluation and Management of Abdominal Compartment Syndrome in the Emergency Department. J Emerg Med. 2019. https://doi.org/10.1016/j.jemermed.2019.09.046
Category: Critical Care
Keywords: pregnancy, peripartum, antepartum, fetal (PubMed Search)
Posted: 12/31/2019 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The arrival of a critically ill pregnant patient to the ED can be anxiety-provoking for emergency physicians as two lives and outcomes must be considered.
Some basic tenets of care, regardless of underlying issue, include:
Finally, once critical illness is identified the OB and NICU teams should be consulted immediately. Fetal distress in a viable pregnancy may be an indication for delivery, and initiation of the transfer process should occur if the supportive specialties are not in-house.
Gaffney A. Critical care in pregnancy: Is it different? Semin Perinatol 2014;38(6):329-40.
Pacheco LD, Saade GR, Hankins GDV. Mechanical ventilation during pregnancy: Sedation, analgesia, and paralysis. Clin Obstet Gynecol 2014;57(4):844-50.
Practice Guidelines of Obstetric Anesthesia: An updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016;124(2):270-300.
Guntupalli KK, Hall N, Karnad D, et al. Critical illness in pregnancy. Chest 2015;148(4):1093-1104.
Category: Critical Care
Keywords: OHCA, cardiac arrest, resuscitation, PEA, pesudo-PEA, pulseless electrical activity (PubMed Search)
Posted: 11/12/2019 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
When managing cardiac arrest, it is important to differentiate PEA, the presence of organized electrical activity without a pulse, from "pseudo-PEA,"where there is no pulse but there IS cardiac activity visualized on ultrasound.
Why:
How:
What:
Bottom Line: Pseudo-PEA is different from PEA. Utilize POCUS during your cardiac arrests to identify it and to help diagnose reversible causes, and treat it as a profound shock state with the appropriate supportive measures, i.e. pressors or inotropy.
Rabjohns J, Quan T, Boniface K, Pourmand A. Pseudo-pulseless electrical activity in the emergency department, an evidence based approach. Am J Emerg Med. 2019. DOI:https://doi.org/10.1016/j.ajem.2019.158503
