Category: Pharmacology & Therapeutics
Keywords: andexanet alfa, 4F-PCC, Kcentra, ICH, thrombosis (PubMed Search)
Posted: 11/13/2025 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
This pearl was adapted from a literature update presented by Castin Schulz, PharmD on November 13, 2025.
A 2025 study in the American Journal of Emergency Medicine provides new real-world data on the two most common reversal agents for factor Xa (fXa) inhibitor-related intracranial hemorrhage (ICH).
This national retrospective cohort study evaluated 350 Veterans who received either andexanet alfa (AA) or 4-factor prothrombin complex concentrate (4F-PCC) for fXa inhibitor-related ICH.
Key Findings (Propensity-Matched Analysis)
Clinical Takeaway
In this study of Veterans with fXa inhibitor-related ICH, andexanet alfa did not improve 90-day mortality compared to 4F-PCC. However, its use was associated with a significantly increased risk of 30-day thrombotic events, particularly ischemic stroke.
This study adds to a growing body of literature questioning the safety profile of AA. The authors conclude that the selection of AA should be carefully weighed against the patient's underlying risk of thrombotic events.
Rech MA, Budde E, Evans CT, et al. Andexanet alfa increases 30-day thrombotic events relative to four-factor prothrombin complex concentrate for factor Xa inhibitors-related intracerebral hemorrhage in veterans. Am J Emerg Med. 2025;97:97-102. doi:10.1016/j.ajem.2025.07.037
Category: Pharmacology & Therapeutics
Keywords: Albuterol, Lactate (PubMed Search)
Posted: 4/10/2025 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
Albuterol, a common bronchodilator used in the treatment of asthma and chronic obstructive pulmonary disease (COPD), can cause a surprising increase of lactate levels. The increase in lactate is usually mild to moderate (typically < 4 mmol/L) and transient. It does not necessarily indicate underlying sepsis, tissue hypoxia, or severe metabolic acidosis.
Mechanism:
Albuterol can cause a transient increase in lactate levels due to its beta-2 agonist effects, which promote glycogenolysis and increase anaerobic metabolism. This can result in elevated lactic acid production, even in the absence of tissue hypoxia or shock.
Timing:
This effect is typically seen within 30 minutes of albuterol administration and can persist for 1-2 hours after discontinuing treatment.
Monitoring:
If lactate levels are elevated in a patient receiving albuterol, consider the possibility of a pharmacologic cause rather than immediately assuming a more serious etiology like shock or severe metabolic disturbance.
Differentiating Causes of Elevated Lactate:
In a critically ill patient, elevated lactate can indicate hypoperfusion (e.g., septic shock, cardiogenic shock, or hypovolemic shock). However, when elevated lactate is associated with albuterol administration, the rise in lactate is often lower and resolves without intervention.
Management:
If albuterol-induced lactate elevation is suspected, continue with supportive care and monitor lactate trends. No specific treatment is necessary for the elevated lactate unless there are other concerning clinical findings that suggest a different underlying cause.
Conclusion:
In emergency settings, it's important to recognize that albuterol can cause a transient increase in lactate levels. Understanding this phenomenon can help avoid misdiagnosis and prevent unnecessary interventions in patients receiving albuterol therapy. Always correlate lactate levels with the broader clinical picture to guide management decisions.
Hockstein M, Diercks D. Significant Lactic Acidosis from Albuterol. Clin Pract Cases Emerg Med. 2018 Mar 14;2(2):128-131. doi: 10.5811/cpcem.2018.1.36024. PMID: 29849230.
Lewis LM, Ferguson I, House SL, Aubuchon K, Schneider J, Johnson K, Matsuda K. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014 Jan;145(1):53-59. doi: 10.1378/chest.13-0930. PMID: 23949578.
Liedtke AG, Lava SAG, Milani GP, Agostoni C, Gilardi V, Bianchetti MG, Treglia G, Faré PB. Selective ß2-Adrenoceptor Agonists and Relevant Hyperlactatemia: Systematic Review and Meta-Analysis. J Clin Med. 2019 Dec 27;9(1):71. doi: 10.3390/jcm9010071. PMID: 31892109.
Maeda T, Paralkar J, Kuno T, Patrawalla P. Inhaled Albuterol Use and Impaired Lactate Clearance in Patients With Sepsis: A Retrospective Cohort Study. J Intensive Care Med. 2021 Mar;36(3):284-289. doi: 10.1177/0885066619901095. Epub 2020 Jan 22. PMID: 31964210.
Zitek T, Cleveland N, Rahbar A, Parker J, Lim C, Elsbecker S, Forred W, Slattery DE. Effect of Nebulized Albuterol on Serum Lactate and Potassium in Healthy Subjects. Acad Emerg Med. 2016 Jun;23(6):718-21. doi: 10.1111/acem.12937. Epub 2016 May 11. PMID: 26857949.
Category: Pharmacology & Therapeutics
Keywords: Antibody-drug conjugates, toxicities, adverse effects (PubMed Search)
Posted: 12/11/2024 by Wesley Oliver
Click here to contact Wesley Oliver
A recent review article highlighted the adverse effects that emergency physicians should know of with the novel antineoplastic agents. The adverse effects and the associated agents are briefly summarized from the article in the table below. A link to the full article is below.
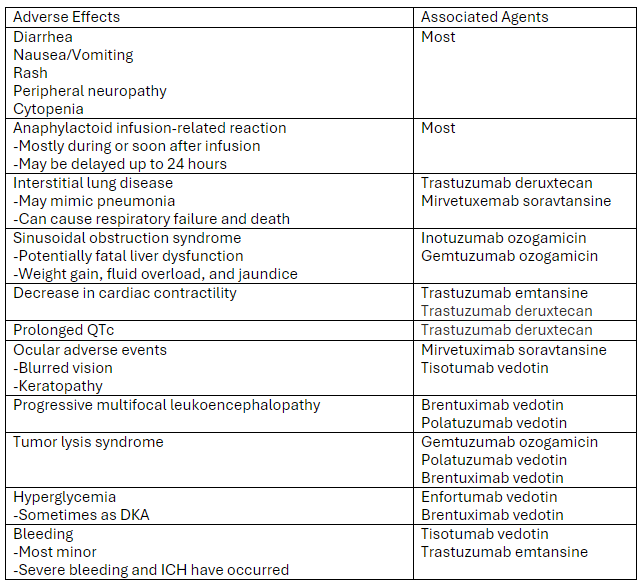
Link to article: Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know - Annals of Emergency Medicine
Markides DM, Hita AG, Merlin J, Reyes-Gibby C, Yeung SJ. Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know. Ann Emerg Med. 2024 Dec 3:S0196-0644(24)01142-9. doi: 10.1016/j.annemergmed.2024.10.015. Epub ahead of print. PMID: 39641680.
Category: Pharmacology & Therapeutics
Keywords: Hyponatremia, Correction, 3% Sodium Chloride, Hypertonic Saline (PubMed Search)
Posted: 7/11/2024 by Wesley Oliver
Click here to contact Wesley Oliver
At our institution we have developed a guideline for the use of hypertonic saline in hyponatremia.
Administration of 3% sodium chloride for acute or symptomatic hyponatremia
Acute hyponatremia with severe symptoms
Acute hyponatremia with moderate symptoms
Hyponatremia Fluid Rate Calculations (**Be Careful with Online Calculators**)
FYI: 3% Sodium Chloride (1.95 mL/mEq; 513 mEq/1 L); 0.9% Sodium Chloride (6.5 mL/mEq; 154 mEq/1 L)
Equations for Calculations
***See Visual Diagnosis for an Example with Calculations***
Example:
70 kg male patient with a current sodium of 115 mEq/L (not hyperglycemic)
3% Sodium Chloride
0.9% Sodium Chloride
**Popular Online Calculator Using Same Example**
3% sodium chloride: 54 mL/hr
0.9% sodium chloride: 551 mL/hr
Be aware that the default setting of the calculator is to correct by 12 mEq/L over 24 hours leading to larger rates of infusion.
Adult Hypertonic Aline for Use in Hyponatremia, Medication Use Guideline. University of Maryland Medical System. Accessed July 2024.
Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines.
JASN. 2017; 28(5):1340-1349.
Jones GN, Bode L, Riha H et al. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care. 2017; 26(1): 37-42.
Sodium chloride preparations. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com. Accessed June 2018.
Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatremia. Intensive Care Med. 2014; 40:320-331.
Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, Evaluation and Treatment of Hyponatremia: Expert Panel Recommendations. Amer J Med. 2013; 126:S1-S42.
Category: Pharmacology & Therapeutics
Keywords: Bactrim, skin and soft tissue infections, Streptococcus spp (PubMed Search)
Posted: 3/14/2024 by Wesley Oliver
Click here to contact Wesley Oliver
MYTH: Bactrim cannot be used as monotherapy for nonpurulent skin and soft tissue infections.
Not True!
Organisms of concern: Streptococcus spp.
Here’s why:
TRUTH: Bactrim CAN be used as monotherapy for nonpurulent skin and soft tissue infections.
Prepared by Rianna Fedora, PharmD on 2/26/24
Category: Pharmacology & Therapeutics
Keywords: DOAC, apixaban, rivaroxaban, loading dose (PubMed Search)
Posted: 9/14/2023 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
DOACs (dabigatran*, apixaban, rivaroxaban) each have different dosing strategies based on indication and patient characteristics. While there is no official term for the doses, the higher initial doses for apixaban (10 mg BID for 7 days) and rivaroxaban (15 mg BID for 21 days) for the treatment of venous thromboembolism (VTE) are commonly referred to as “loading doses.” However, the term “loading dose” is actually a misnomer.
Loading doses are used to reach therapeutic drug levels quicker with medications such as vancomycin and phenytoin/fosphenytoin. However, this is not the purpose of the higher initial doses of apixaban and rivaroxaban. The purpose of the higher doses is to provide increased levels of anticoagulation during the acute phase of VTE when patients are hypercoagulable. For this reason, VTE and heparin-induced thrombocytopenia are the only indications where a higher dose is used initially, all other indications start with the standard dose. The difference in duration of these higher doses between apixaban (7 days) and rivaroxaban (21 days) are due to the durations used in trials by the drug company, versus any pharmacokinetic reasons.
To apply this concept:
Apixaban/Rivaroxaban: For the treatment of VTE, a higher dose is only required for the initial 7- (apixaban) or 21-day period (rivaroxaban). After this period, if there is any interruption in therapy, the standard dose can be restarted because therapeutic levels are rapidly achieved and higher doses are not needed outside of the acute phase.
One caveat to this would be if the patient developed a new VTE while therapy is interrupted, in which case another period of the higher dosing could be considered.
*Remember: Dabigatran cannot be used for initial treatment of VTE and must be started only after at least 5 days of a parenteral anticoagulant. (Dabigatran and the parenteral anticoagulant should not be overlapped).
References
Eliquis (apixaban) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; April 2021.
Pradaxa (dabigatran) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; June 2021.
Xarelto (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals Inc; February 2023.
Category: Pharmacology & Therapeutics
Keywords: Tenecteplase, Pulmonary Embolism, Cardiac Arrest (PubMed Search)
Posted: 8/10/2023 by Wesley Oliver
Click here to contact Wesley Oliver
ACLS guidelines state that thrombolytics may be considered for suspected pulmonary embolism during cardiac arrest. There is limited data supporting the recommendation; however, it is noted that the benefits likely outweigh the risks. There is also no consensus on the appropriate thrombolytic timing, drug, or dose.
Our institution recently implemented the use of tenecteplase for acute ischemic stroke, ST-elevation myocardial infarction (STEMI), and pulmonary embolism (PE). When using tenecteplase for suspected PE during cardiac arrest, we use the same weight-based dose used for STEMIs. We include a label on the outside of the tenecteplase box that lists all the doses for the various indications.
Tenecteplase Dose
<60 kg: 30 mg
≥60 to <70 kg: 35 mg
≥70 to <80 kg: 40 mg
≥80 to <90 kg: 45 mg
≥90 kg: 50 mg
The tenecteplase dose is administered as an IV bolus over 5 seconds.
There is also limited data for the duration of CPR after thrombolytic administration, with no recommendations being made in most literature. Our current institutional guidelines recommend to consider continuing CPR for 60-90 minutes before resuscitation efforts are terminated. The only guideline that makes any mention of duration of CPR is the European Resuscitation Council Guidelines 2021, which makes the same recommendation.
Berg KM, Soar J, Andersen LW, et al. Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2020; 142(suppl 1):S92-139.
Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132(suppl 2):S501-S518.
Lott C, Truhlá A, Alfonzo A, et al; ERC Special Circumstances Writing Group Collaborators. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation. 2021 Apr;161:152-219.
TNKase (tenecteplase) [prescribing information]. South San Francisco, CA: Genentech; March 2023.
Category: Pharmacology & Therapeutics
Keywords: Calcium, Massive transfusion protocol, Citrate, Blood products (PubMed Search)
Posted: 7/13/2023 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
Citrate is an anticoagulant added to blood products to maintain stability for storage. With the administration of large volumes of blood products, citrate binds to ionized calcium, which can cause hypocalcemia. Evidence for specific calcium administration during massive transfusion protocols is limited; however, a proposed strategy has been to administer calcium gluconate 2 grams for every 2-4 units of red blood cells.
Robinson, et al. performed a retrospective analysis attempting to determine the optimal Citrate:Ca ratio (a novel ratio created for this study) to reduce 30-day mortality. They did not find any differences in mortality; however, they found a Citrate:Ca ratio of 2-3 produced a normalized ionized calcium level with 24 hours of a massive transfusion protocol.
Based on their calculations, this would equate to supplementing 1 g of calcium gluconate for every 3 units of red blood cells given.
***Reminder: Based on the amount of elemental calcium in each gram of calcium gluconate (4.7 mEq) and calcium chloride (13.6 mEq); 3 g calcium gluconate=1 g calcium chloride.***
Bottom Line: Supplementing with calcium gluconate 1 g for every 3 units of red blood cells should be sufficient to maintain normal ionized calcium levels after a massive transfusion protocol.
Robinson A, Rech MA, DeChristopher PJ, Vaughn A, Rubino J, Bannister E, Moore ME, Chang K. Defining the optimal calcium repletion dosing in patients requiring activation of massive transfusion protocol. Am J Emerg Med. 2023 May 13;70:96-100.
Category: Pharmacology & Therapeutics
Keywords: Angioedema, ACE-inhibitor, C1-Esterase Inhibitor, ACEi, C1INH, Berinert (PubMed Search)
Posted: 2/3/2023 by Wesley Oliver
(Updated: 2/4/2023)
Click here to contact Wesley Oliver
ACE-inhibitor (ACEi) induced angioedema is mediated by bradykinin and there are no proven medications for the treatment of this disease. Theoretically, a C1-esterase inhibitor (C1INH) could be beneficial; however, data has not demonstrated any efficacy for these agents.
Strassen et al. recently published a double-blind, randomized, controlled, multicenter trial of 30 patients comparing C1NH (Brand Name: Berinert) to placebo. In addition to standard treatment, a dose of C1INH (Berinert) 20 IU/kg or placebo (0.95% NaCl) was administered intravenously.
The primary endpoint was the time to complete resolution of signs and symptoms of edema (TCER). When compared to placebo, the original primary analysis demonstrated that the placebo arm (15 hours) resolved faster than the C1INH arm (24 hours, p=0.046).
This study is further evidence against the use of C1INH for ACE-inhibitor induced angioedema. The primary focus in the treatment of ACEi induced angioedema should continue to be airway management.
For reference, at our institution we have both C1INH (Berinert) and icatibant on formulary and they are restricted to only being used for acute hereditary angioedema attacks and cannot be used for ACEi induced angioedema.
Strassen U, et al. Efficacy of human C1 esterase inhibitor concentrate for treatment of ACE-inhibitor induced angioedema. Am J Emerg Med. 2023;64:121-128.
Wilkerson RG, Martinelli AN, Oliver WD. Treatment of angioedema induced by angiotensin-converting enzyme inhibitor [letter]. J Emerg Med. 2018;55:132-133.
Category: Pharmacology & Therapeutics
Keywords: Intaosseous, Pharmacy, Medications (PubMed Search)
Posted: 9/3/2022 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
Intraosseous (IO) administration uses bone marrow to deliver fluids and medications during cardiac resuscitation or other emergent situations where IV access cannot be established.
IV versus IO
Considerations When Using IO Access
References
Dornhofer P, Kellar JZ. Intraosseous Vascular Access. [Updated 2022 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554373/
Von Hoff DD, Kuhn JG, Burris HA 3rd, Miller LJ. Does intraosseous equal intravenous? A pharmacokinetic study. Am J Emerg Med. 2008;26(1):31-38. doi:10.1016/j.ajem.2007.03.024
Langley DM, Moran M. Intraosseous needles: they're not just for kids anymore. J Emerg Nurs. 2008;34(4):318-319. doi:10.1016/j.jen.2007.07.005
Ngo AS, Oh JJ, Chen Y, Yong D, Ong ME. Intraosseous vascular access in adults using the EZ-IO in an emergency department. Int J Emerg Med. 2009;2(3):155-160. Published 2009 Aug 11. doi:10.1007/s12245-009-0116-9
Category: Pharmacology & Therapeutics
Keywords: Droperidol, QTc (PubMed Search)
Posted: 5/7/2022 by Wesley Oliver
Click here to contact Wesley Oliver
A recent prospective cohort study investigated the effect of low-dose droperidol on QTc in an emergency department:
Low-dose droperidol has a small effect on QTc and most patients remained below 500 ms.
Hernández-Rodríguez L, Bellolio F, Cabrera D, et al. Prospective real-time evaluation of the QTc interval variation after low-dose droperidol among emergency department patients. Am J Emerg Med. 2022 Feb;52:212-219.
Category: Pharmacology & Therapeutics
Keywords: MIS-C, COVID (PubMed Search)
Posted: 2/7/2022 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
Background:
Multisystem inflammatory syndrome in children (MIS-C) as defined by CDC Health Advisory in May 2020 is:
1) An individual aged <21 years presenting with fever*, laboratory evidence of inflammation**, and evidence of clinically severe illness requiring hospitalization, with multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological); AND
2) No alternative plausible diagnoses; AND
3) Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to the onset of symptoms.
*Fever >38.0°C for ≥24 hours, or report of subjective fever lasting ≥24 hours
**Including, but not limited to, one or more of the following: an elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, d-dimer, ferritin, lactic acid dehydrogenase (LDH), or interleukin 6 (IL-6), elevated neutrophils, reduced lymphocytes and low albumin
As of January 31st, 2022 the CDC reports the following statistics related to MIS-C in the United States:
· Total MIS-C patients meeting case definition= 6,851
· Total MIS-C deaths meeting case definition = 59
· The median age of patients with MIS-C was 9 years. Half of children with MIS-C were between the ages of 5 and 13 years.
· 59% of the reported patients with race/ethnicity information available occurred in children who are Hispanic/Latino (1,746 patients) or Black, Non-Hispanic (2,050 patients).
· 98% of patients had a positive test result for SARS CoV-2, the virus that causes COVID-19. The remaining 2% of patients had contact with someone with COVID-19.
· 60% of reported patients were male.
Management:
First-Line Treatment:
· IVIG 2 g/kg dosed based on ideal body weight with a maximum of 100 grams (1000 mL)
o For patients with significant myocardial dysfunction and concern for fluid overload, the infusion can be given in divided doses over 2 days (1g/kg q12 x 2 doses)
PLUS
· Methylprednisolone 1 mg/kg (max of 30 mg/dose) IV twice daily and switch to PO and taper when clinically appropriate
Upon Consultation with Pediatric Hematology/Cardiology will consider adding the following therapies to IVIG and steroids:
· Enoxaparin treatment versus prophylactic dosing depending on D-dimer elevation and whether or not being admitted to PICU
· Aspirin 3-5 mg/kg (max 81 mg/dose) daily unless platelet count < 80 K/mcl
Second-Line Treatment (refractory to IVIG defined by symptoms and fever persisting >36 hours)*:
· Methylprednisolone pulse dosing- 30 mg/kg (max of 1000 mg/dose) x 3-5 days
OR
· High dose anakinra
OR
· Infliximab 5-10 mg/kg IV x1
*All second-line treatment options require peds infectious diseases and PICU attending approval
UMMS COVID/MIS-C Pathway: https://intra.umms.org/-/media/intranets/umms/pdfs/dept/pharmacy-and-therapeutics/guidelines/umms-pediatric-covid-pathway.pdf?upd=20220125144550
References:
1. Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic external icon. Circulation 2020.
2. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic external icon. Lancet 2020.
3. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study external icon. Lancet 2020.
4. CDC COVID Data Tracker: Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. February 1, 2022.
5. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis and Rheumatology 2022.
Category: Pharmacology & Therapeutics
Keywords: Kcentra, AC Reversal, Anticoagulant (PubMed Search)
Posted: 11/6/2021 by Wesley Oliver
Click here to contact Wesley Oliver
Kcentra (four-factor prothrombin complex concentrate, 4f-PCC) is approved for the reversal of warfarin using a weight-based dosing strategy based on INR. However, since the approval of Kcentra, data has shown a fixed-dose strategy and use for direct-acting oral anticoagulants (DOAC) is appropriate. There are even recommendations to use a fixed-dose for DOACs in some situations. Utilizing a fixed-dose strategy can help with decreasing drug preparation/delivery times and costs.
Our institution now only uses a weight-based Kcentra dose of 50 units/kg for patients on DOACs with ICH or trauma-induced coagulopathy. All other patients receive a fixed-dose of Kcentra 1,500 units or 2,000 units based on anticoagulant and other criteria.
Below is a diagram summarizing our current dosing strategy for Kcentra at our institution.
ICH=intracerebral hemorrhage
DOAC=direct-acting oral anticoagulant (rivaroxaban, apixaban, and edoxaban)
Other points of interest at our institution:
Kcentra® [package insert]. CSL Behring, Marburg, Germany; 2013.
https://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf
Tomaselli GF, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020 Aug 4;76(5):594-622.
University of Maryland Medical Center. 2021. Pre- and Peri-Procedural Management of Anticoagulation, Management of Bleeding in the Setting of Anticoagulation, Intracranial Hemorrhage, and Dilutional Coagulopathies. Internal guideline.
Category: Pharmacology & Therapeutics
Keywords: Gonococcal Infections, Ceftriaxone, Doxycycline, Azithromycin, CDC (PubMed Search)
Posted: 7/3/2021 by Wesley Oliver
Click here to contact Wesley Oliver
| Uncomplicated Gonococcal Infections | 2015 Recommendations [1] | 2020 Recommendations [2] |
| Cervical, urethral, rectal, and pharyngeal infection | Ceftriaxone 250 mg IM x 1 dose, plus azithromycin 1 g PO x 1 dose | Ceftriaxone 500 mg IM x 1 dose |
| >=150 kg | No recommendation | Ceftriaxone 1 g IM x 1 dose |
| If coinfection with chlamydia cannot be excluded | Coverage provided by gonococcal treatment regimen | Add doxycycline 100 mg PO BID x 7 days |
1. MMWR Morb Mortal Wkly Rep. 2015;64(3).
2. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911-16.
3. Chisholm SA, et al. J Antimicrob Chemother. 2010;65:2141-48.
4. Connolly KL, et al. Antimicrob Agents Chemother. 2019;63:e01644-18.
5. Duke-Muijrers N, et al. Clin Infect Dis. 2019;69(11):1946-54.
6. Mizushima D, et al. J Antimicrob Chemother. 2021;76:495-98.
Category: Pharmacology & Therapeutics
Keywords: Pyelonephritis, Outpatient, Fluoroquinolones, TMP-SMX, Beta-lactams (PubMed Search)
Posted: 4/3/2021 by Wesley Oliver
Click here to contact Wesley Oliver
While fluoroquinolones have fallen out of favor for many indications due to the ever growing list of adverse effects, they still play an important role in the outpatient treatment of pyelonephritis. Fluoroquinolones and TMP-SMX are the preferred agents due to higher failure rates with beta-lactams.
Preferred Therapies:
Ciprofloxacin 500 mg PO BID*
Levofloxacin 750 mg PO daily*
TMP-SMX 1 DS tab PO BID**
*Consider a single dose of long-acting parenteral agent, such as ceftriaxone, if community prevalence of fluoroquinolone resistance >10%.
**Consider a single dose of long-acting parenteral agent, such as ceftriaxone, if using TMP-SMX.
Alternative Therapies#:
Cefpodoxime 200 mg PO BID
Cefdinir 300 mg PO BID
#Beta-lactams are not preferred agents due to higher failure rates when compared to fluoroquinolones. Consider a single dose of long-acting parenteral agent, such as ceftriaxone, if using beta-lactams.
Duration of Therapy: 10-14 days
Take Home Point:
Utilize ciprofloxacin, levofloxacin, or TMP-SMX over beta-lactams when discharging patients with oral antibiotics for pyelonephritis.
Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103.
Urinary Tract Infections. UMMS Clinical Practice Guidelines. Sanford Guide, 2021. Accessed April 2, 2021. https://webedition.sanfordguide.com/en/umms/syndromes/urinary-tract-infections.
Category: Pharmacology & Therapeutics
Keywords: Buprenorphine, Acute Pain (PubMed Search)
Posted: 2/6/2021 by Wesley Oliver
Click here to contact Wesley Oliver
Buprenorphine is a partial opioid receptor agonist that has a higher binding affinity than pure opioid agonists. There can be unease in managing acute pain in patients sustained on buprenorphine for opioid use disorder due to many factors.
The main barriers to effective pain management in these patients are:
Take Home Points
In general, the treatment strategy for acute pain in patients on buprenorphine should be:
Category: Pharmacology & Therapeutics
Keywords: Octreotide, Vasopressin, Variceal Bleeding (PubMed Search)
Posted: 1/2/2021 by Wesley Oliver
Click here to contact Wesley Oliver
With a national shortage of octreotide an alternative treatment plan had to be implemented at our institution for patients presenting with variceal bleeding.
Drug references recommend a continuous infusion of vasopressin at 0.2 to 0.4 units/minute. Dose may be titrated as needed to a maximum dose of 0.8 units/minute with maximum duration of 24 hours to reduce incidence of adverse effects. Administer IV nitroglycerin concurrently to prevent ischemic complications and monitor closely for signs/symptoms of myocardial, peripheral, and bowel ischemia.
Protocol at our institution:
Vasopressin
Initiate vasopressin at 0.2 units/min.
Increase by 0.2 units/min if bleeding is not controlled after one hour (max dose: 0.8 units/min).
If bleeding controlled for 2 hours, can decrease by 0.2 units/min and reassess.
Limit use to 24 hours.
Nitroglycerin
Use nitroglycerin infusion to prevent adverse effects from vasopressin.
Initiate nitroglycerin at 40 mcg/min, titrate by 40 mcg/min to a max dose of 400 mcg/min.
Goal systolic blood press pressure of 90-100 mmHg. Do not start nitroglycerin if SBP <90 mmHg.
***Please note the vasopressin dose for this indication is significantly higher than the typical dose of 0.03 units/min we use for shock.***
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases [published correction appears in Hepatology. 2017;66(1):304]. Hepatology. 2017;65(1):310-335.
Terés J, Planas R, Panes J, Salmeron JM, Mas A, Bosch J, Llorente C, Viver J, Feu F, Rodés J. Vasopressin/nitroglycerin infusion vs. esophageal tamponade in the treatment of acute variceal bleeding: a randomized controlled trial. Hepatology. 1990 Jun;11(6):964-8.
Vasopressin. Lexicomp. UpToDate. Waltham, MA: UpToDate Inc. Available at: https://www.uptodate.com. Accessed on December 31, 2020.
Category: Pharmacology & Therapeutics
Keywords: Beta-Lactam, Allergies, Sepsis (PubMed Search)
Posted: 11/7/2020 by Wesley Oliver
Click here to contact Wesley Oliver
Komyathy KL, Judd WR, Ratliff PD, Hughes RE. Assessing mortality outcomes of beta-lactam-allergic patients presenting with sepsis. Am J Emerg Med. 2020;38(9): 1816-1819.
Category: Pharmacology & Therapeutics
Keywords: Cirrhosis, Pain, Acetaminophen, NSAID, Opioid (PubMed Search)
Posted: 8/1/2020 by Wesley Oliver
(Updated: 2/8/2026)
Click here to contact Wesley Oliver
The liver performs an essential role in the metabolism and clearance of many drugs. Liver damage due to cirrhosis can decrease first-pass metabolism of oral medications and increase free-drug concentrations of protein-bound medications due to decreased albumin production. In the absence of cirrhosis, patients with chronic hepatitis or hepatic cancer may only have a small decrease in drug clearance. Hepatic dose adjustments are not as prevalent or readily available as renal dose adjustments, which can create difficulty in finding the balance between pain relief and adverse effects.
The most common medications used for pain control in the emergency department are acetaminophen, NSAIDs, and opioids.
Acetaminophen
It is sometimes misconceived that acetaminophen should never be used in patients with cirrhosis due to the common knowledge that acetaminophen overdoses can cause hepatotoxicity. Alcoholics may have an increased risk of hepatotoxicity due to induction of CYP2E1 and decreased glutathione stores. However, acetaminophen is safe in patients with cirrhosis when used at appropriate doses. Limit the total daily dose of acetaminophen to 2 g daily in patients with cirrhosis and avoid acetaminophen in patients that are actively drinking. Also, educate patients that over-the-counter (OTC) and prescription medications may contain acetaminophen.
NSAIDs
In patients with cirrhosis, NSAIDs have increased bioavailability due to decreased CYP metabolism and decreased protein binding. In addition, prostaglandin inhibition can precipitate renal failure and sodium retention, worsening ascites and increasing the risk of hepatorenal syndrome, and increase the risk of gastrointestinal bleeding. Thrombocytopenia from NSAID use can further increase the risk of bleeding. Thus, avoid NSAID use in patients with cirrhosis. Topical NSAIDs can be considered.
Opioids
Opioid metabolism is altered in patients with cirrhosis and can contribute to complications with cirrhosis, such as precipitating encephalopathy. Generally, the bioavailability is increased and half-life is extended; thus, lower doses of immediate-release (IR) formulations at extended dosing intervals should be utilized. Common opioids for acute pain control in the emergency department are fentanyl, hydrocodone/oxycodone, hydromorphone, and morphine.
Take Home Points
| Drug/Class | Preferred Agent | Considerations |
| Acetaminophen | Max daily dose 2 g/day | Avoid if actively drinking. Be cautious if patient also taking OTC or combination products. |
| NSAIDs | None; Avoid | Topical NSAIDs may be considered. |
| Opioids | Hydromorphone, Fentanyl | Start with IR products at lower doses and extended intervals. |
1. Rakoski M, Goayl P, Spencer-Safier M, Weissman J, Mohr G, Volk M. Pain management in patients with cirrhosis. Clinical Liver Disease. 2018;11:135-140.
2. Wehrer M. Pain management considerations in cirrhosis. US Pharm. 2015;40:HS5-HS11.
Category: Pharmacology & Therapeutics
Keywords: Metronidazole, Disulfiram-like Reaction (PubMed Search)
Posted: 6/6/2020 by Wesley Oliver
Click here to contact Wesley Oliver
While taking metronidazole it is advised that patients avoid ethanol use for at least 3 days after therapy due to the possibility of a disulfiram-like reaction. The disulfiram-like reaction presents as abdominal cramps, nausea, vomiting, headaches, and/or flushing and can cause extreme discomfort for patients. A recent case report describes a case of a disulfiram-like reaction in a patient receiving metronidazole and an oral prednisone solution that contained 30% alcohol. This case highlights an important point. Not only should we counsel patients about avoiding alcoholic beverages for at least 3 days after metronidazole therapy, but they should also avoid all alcohol-containing products, such as oral solutions and mouthwash.