Category: Critical Care
Keywords: massive transfusion, bleeding (PubMed Search)
Posted: 9/23/2014 by Feras Khan, MD
(Updated: 2/8/2026)
Click here to contact Feras Khan, MD
What is a massive transfusion?
When would I use this?
Indications:
-Systolic Blood pressure < 100
-Unable to obtain blood pressure
AND
-Penetrating torso trauma
-Positive FAST
-External blood loss
-Plans to go to the OR
How do I give it?
Does this apply for just traumatic bleeding?
Are there other agents I can use?
What am I trying to do with this protocol?
Murthi SB, Stansbury LG, Dutton RP, et al. TRAnsfusion medicine in trauma patients: an update. Expert Rev Hematol. 2011 Oct;4(5):527-37.
Hess JR, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008 Oct; 65(4):748-54.
University of Maryland SHOCK Trauma Massive Transfusion Protocol. 2011.
Category: Critical Care
Posted: 9/16/2014 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Infectious Risks Associated with TTM
Kuchena A, et al. Postcardiac arrest temperature management: infectious risks. Curr Opin Crit Care 2014; 20:507-15.
Category: Critical Care
Posted: 9/8/2014 by John Greenwood, MD
(Updated: 9/9/2014)
Click here to contact John Greenwood, MD
Goal-Directed Resuscitation During Cardiac Arrest
Focusing on high-quality CPR is by far one of the most effective methods to ensure your arrested patient has the best chance to survive. However, emerging evidence suggests that there are additional goals that we should try and accomplish during our resuscitation.
As we continue to move toward goal-directed resuscitation strategies, optimizing coronary perfusion pressure (CPP) may be our next target in “personalizing” the care we provide to those in cardiac arrest.
A recent AHA consensus statement recommended the following physiologic goals during cardiac arrest care:
Each of these variables can give the provider valuable feedback about how their patient is responding to their resuscitation. Some argue that the DBP target should be much higher (>35 mmHg), with the caveat that pharmacologic optimization can only occur once high quality CPR is confirmed. The goal should always be to minimize the use of epinephrine whenever possible!
Bottom Line: During your next cardiac arrest resus, consider using a goal-directed strategy by monitoring the patient’s CPP, DBP, & EtCO2 to determine the effectiveness of your resuscitation.
Suggested Reading
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 9/2/2014 by Haney Mallemat, MD
(Updated: 10/1/2014)
Click here to contact Haney Mallemat, MD
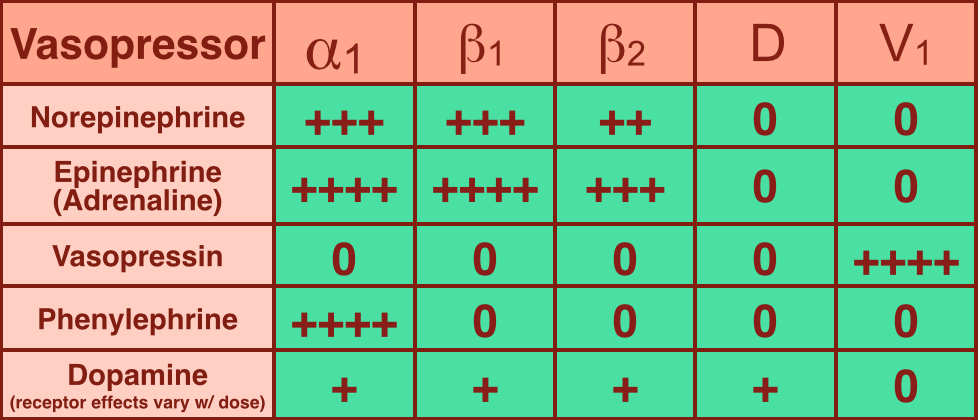
Vasopressors are used in shock-states to increase mean arterial pressure (MAP) and improve distal tissue perfusion. Additionally, some agents have effects on the heart to augment cardiac output.
Receptors that vasopressors work on include:
Norepinephrine (NE): excellent vasopressor for most types of shock and recommended as a first-line agent in the Surviving Sepsis Guidelines.
Epinephrine (a.k.a. Adrenaline): in several countries the first-line agent for shock (including sepsis).
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: immunonutrition, enteral feeding (PubMed Search)
Posted: 8/26/2014 by Feras Khan, MD
(Updated: 2/8/2026)
Click here to contact Feras Khan, MD
Background
Data
What to do
Category: Critical Care
Posted: 8/19/2014 by Mike Winters, MBA, MD
(Updated: 2/8/2026)
Click here to contact Mike Winters, MBA, MD
Sepsis Pearls from the Recent Literature
Category: Critical Care
Keywords: cardiomyopathy, sepsis, septic shock, pressors, inotropes, epinephrine, norepinephrine, dobutamine (PubMed Search)
Posted: 8/12/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Should I Give My Patient with Septic Cardiomyopathy Fluids?
The incidence of acute LV dysfunction in septic shock is estimated to occur in 18 - 46% of patients within the first 24 hours of shock. Unlike the "classic" pattern of cardiogenic shock where LV filling pressure is high, in septic shock there are normal or low LV filling pressures.
Three therapeutic options should be strongly considered in the patient with a septic cardiomyopathy [CM]:
Recommended Reading
Vieillard-Baron, A. Septic cardiomyopathy. Ann Intensive Care. 2011; 1:6.
Follow me on Twitter @JohnGreenwoodMD
For more critical care pearls & education check out http://www.marylandccproject.org
Category: Critical Care
Posted: 8/5/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
There are many ventilator modes to choose from, but almost every mode can be distilled down to its basic principles by understanding the “Three T's of Mechanical Ventilation”
Trigger: You must determine whether the vent or patient will trigger a mechanical breath. For example, machine-triggered breaths (a.k.a. control mode of ventilation) are used for paralyzed patients and will deliver a breath after a period of time has elapsed (e.g., if RR is 10/min, then a breath is given every 6 seconds). On the other hand, if a patient’s respiratory drive is intact (a.k.a. assist-mode) than the patient triggers the breath when the vent detects a patient induced change in airflow or airway pressure. These two modes can also be mixed together.
Target: Mechanical breaths must have a specific target, either a target airway pressure or a tidal volume. Because pressure and volume are directly related, pick the variable you want to target and the other parameter will vary depending on the patient’s intrinsic physiology. For example, if you choose to target a specific tidal volume, we may get one plateau pressure in a patient with normal lungs, but a higher plateau pressure in another patient with stiffer lungs.
Terminate: You must decide when the mechanical breath (i.e., inspiration) terminates and expiration begins. Termination occurs: 1) after a set inspiratory time has elapsed in certain pressure-targeted modes, 2) when a predefined target volume has been achieved (i.e., volume-cycled modes), or 3) when airflow has been reduced by a certain percentage (as in pressure-support ventilation; to be discussed separately)
Let’s put this all together by looking at an example: pressure control ventilation (rate = 12/min and target pressure 20cm H20). Trigger: Because this is a “control”, not assist mode, the machine will trigger a breath 12 times per minute or every 5 seconds. Target: Here we chose to have pressure be the target, so when the ventilator triggers a breath it will deliver a constant airway pressure of 20 cmH2O until we tell the vent terminate that breath. Terminate: the constant airway pressure will be turned off after a fixed period of time has elapsed; for this example we will set the inspiratory time as 1 second, then expiration begins. Now, after a few vent breaths we will observe the results of our settings and reassess; if the resulting tidal volume is lower than what we wanted, we will increase the target pressure to increase the tidal volume. If the tidal volume is higher than what we wanted, we will reduce the target pressure to reduce the tidal volume. We can also tweak the inspiratory time to manipulate the tidal volume, but this does so to a lesser degree.
Try to break down your favorite modes of ventilation using the Three T’s and see if this helps you understand vent modes better.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: epinephrine, im, anaphylaxis, allergic reaction, observation (PubMed Search)
Posted: 7/29/2014 by Feras Khan, MD
Click here to contact Feras Khan, MD
Observation after giving IM Epi for allergic reactions or anaphylaxis
Background
Question
Meta-analysis
Results
Limitations
What to do?
Grunau B, et al. Incidence of Clinically Important Biphasic Reactions in Emergency Department Patients with Allergic Reactions or Anaphylaxis. Annals of Emergency Medicine. Vol 63, No 6; June 2014 736-743.
Category: Critical Care
Posted: 7/22/2014 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Predicting Neurologic Outcome in Patients Treated with TTM
Golan E, et al. Predicting neurologic outcome after targeted temperature management for cardiac arrest: Systematic review and meta-analysis. Crit Care Med 2014; 42:1919-30.
Category: Critical Care
Posted: 7/14/2014 by John Greenwood, MD
(Updated: 7/15/2014)
Click here to contact John Greenwood, MD
Patient Positioning During Mechanical Ventilation
In any patient with acute respiratory failure, it is extremely important to consider patient positioning after initiating mechanical ventilation. Both ventilation (V) and perfusion (Q) of the lungs can be significantly altered by manipulating the way you position your patient.
Follow me on Twitter @JohnGreenwoodMD
For more critical care pearls & education check out http://www.marylandccproject.org
Category: Critical Care
Posted: 7/8/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Editors note: The new Back 2 Basic series will review essential critical care concepts on the first Tuesday of each month. Want a specific topic reviewed? Contact us by email or Twitter.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: blood, anemia, infection, blood transfusions (PubMed Search)
Posted: 7/1/2014 by Feras Khan, MD
(Updated: 2/8/2026)
Click here to contact Feras Khan, MD
Risk of infection from Blood transfusions
JAMA Meta-Analysis
What they found
Bottom Line
Rohde J, et al. Health Care Associated Infection after Red Blood Cell Transfusion. A systematic Review adn Meta-Analysis. JAMA 2014; 311(13): 1317-1326.
Category: Critical Care
Posted: 6/24/2014 by Mike Winters, MBA, MD
(Updated: 2/8/2026)
Click here to contact Mike Winters, MBA, MD
Prophylactic FFP for Procedures?
Category: Critical Care
Keywords: Thrombelastography, TEG, ROTEM, Hemorrhagic Shock (PubMed Search)
Posted: 6/13/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Thrombelastography for Management of Non-Traumatic Hemorrhagic Shock
The use of thrombelastography (TEG, ROTEM) has traditionally been utilized and studied in the management of acute coagulopathy of trauma (ACoT) developed by patients in hemorrhagic shock secondary to trauma.
Functional coagulation tests such as the TEG may provide valuable information when resuscitating the hemorrhaging patient, especially if there is any concern for an underlying coagulopathy.
The following is a TEG recently returned during the resuscitation of a 60 y/o male with a history of HCV cirrhosis presenting with hemorrhagic shock secondary to a massive upper GIB. The University's Massive Transfusion Protocol was promptly activated and at this point, the patient had received approximately 4 units of PRBCs & FFP along with 1 liter of crystalloid. His Hgb was 5, PT/PTT/INR were undetectable, and his fibrinogen was 80.
Below is a table that simplifies the treatment, based on the test's abnormalities:
After reviewing the initial TEG, all perameters were abnormal in addition to the presence of significant fibrinolysis. The patient was given an additional 4 units of FFP, DDAVP, cryoprecipitate, a unit of platelets, and aminocaproic acid. The patient still required significant resuscitation, however bleeding had significantly decreased as well has his pressor requirement. Below is the patient's follow-up TEG 2 hours later.
There is growing enthusiasm for the use of functional coagulopathy testing in the patient with hemorrhagic shock. Early resuscitation with blood products as your fluid of choice with limited fluid administration while arranging for definitive source control are critical, but also consider early thrombelastography to detect additional causes for uncontrolled hemorrhage.
References
Follow Me On Twitter: @JohnGreenwoodMD
email: johncgreenwood@gmail.com
Category: Critical Care
Posted: 6/10/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: bleeding, coagulopathy, dabigatran, PCC, (PubMed Search)
Posted: 6/3/2014 by Feras Khan, MD
(Updated: 2/8/2026)
Click here to contact Feras Khan, MD
Emergent reversal of Dabigatran
What is it:
Direct thrombin inhibitor used for stroke prevention in non-valvular atrial fibrillation
When do I worry about reversal:
Patients can have clinically important bleeding (GI hemorrhage, or Intracranial bleeding) or need reversal for emergent surgery
Patients with renal failure can have a prolonged medication effect
What can I do:
1. Activated charcoal: good for recent overdose or recent ingestion (within 2 hours)
2. Hemodialysis: around 60-65% can be removed within 2-4 hrs; putting in a dialysis line can be…bloody
3. FFP: in rat studies, has been shown to reduce the volume of intracranial hemorrhage. Unknown in humans. No good evidence of use based on coagulation mechanisms. Still worth a try though.
4. Recombinant activated factor VII: Has been shown to correct the bleeding time in animal studies. Probably the best bet in severe bleeding
5. Pro-thrombin complex concentrate: has been shown to decrease the bleeding time in animal studies
How do I monitor effect?
No great way here. Check aPTT and thrombin time (TT). At supra-therapeutic doses there is no good test.
Coming attractions: Dabigatran-fab for emergent reversal (see previous pearl: https://umem.org/educational_pearls/2415/)
Kaatz, S et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. American Journal of Hematology. 2012.
Category: Critical Care
Posted: 5/27/2014 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Are Intermediate Lactate Levels Concerning in Patients with Suspected Infection?
Puskarich MA, et al. Prognosis of emergency department patients with suspected infection and intermediate lactate levels: A systematic review. J Crit Care 2014; 29:334-339
Category: Critical Care
Keywords: Carbapenem Resistant Organisms, CRE, Pseudomonas, Infectious Diseases, Antimicrobial Stewardship (PubMed Search)
Posted: 5/15/2014 by John Greenwood, MD
(Updated: 5/20/2014)
Click here to contact John Greenwood, MD
We've all heard Dr. Bryan Hayes warn us that, "Vanc & Zosyn is NOT the Answer for Everything" but things just got a little more serious, on a whole 'nother level...
Within the past few months, 2 cases of NDM-producing carbapenem-resistant pseudomonas have been reported in the area - one in Delaware and one in Pennsylvania. Previously, the only reported cases were found in Europe.
Few treatment options are currently available for carbapenem resistant organisms.
Appear to have retained some in vitro activity against these organisms, but are generally used as, "drugs of last resort".
Know it exists, take a good history, & know your local antibiogram. Prior to selecting a broad spectrum antimicrobial regimen, try to obtain previous antimicrobial culture data for patients with resistant organism infectious risk factors.
For more critical care pearls, follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 5/13/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Prior literature has demonstrated the safety and feasibility of placing subclavian lines with ultrasound guidance; here's a link to a short educational video describing the technique.
The literature has been varied, however, as to which approach is best for venous cannulation with ultrasound; the supraclavicular (SC) or infraclavicular (IC) approach (see references below)
A recent study evaluated both approaches in healthy volunteers in order to determine which approach is superior for cannulation using ultrasound.
98 patients were prospective evaluated by Emergency Medicine physicians with training in ultrasound. In each patient, both SC and IC views were evaluated on both the left and right sides; each view was given a grade for ease of favorability (no patients were actually cannulated)
Overall, it was found that the SC view was significantly more favorable compared to the IC view; the right SC was non-significantly preferred compared to the left SC.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Stachura, M. et al. A Comparison of the Supraclavicular and Infraclavicular Views for Imaging the Subclavian Vein with Ultrasound. The American Journal of Emergency Medicine (in press)
Fragou, M., Gravvanis, A., and Vasilios, D. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: A prospective randomized study. Crit Care Med. 2011; 39: 1607–1612
Mallin, M., Louis, H., and Madsen, T. A novel technique for ultrasound-guided supraclavicular subclavian cannulation. Am J Emerg Med. 2010; 28: 966–969
Czarnik, T., Gawda, R., Perkowski, T. et al. Supraclavicular approach is an easy and safe method of subclavian vein catheterization even in mechanically ventilated patients. analysis of 370 attempts. Anesthesiology. 2009; 111:334–339
