Category: Critical Care
Keywords: DDAVP, desmopressin, ICH, intracranial hemorrhage, stroke, CVA, hyponatremia (PubMed Search)
Posted: 12/8/2019 by Robert Brown, MD
(Updated: 12/10/2019)
Click here to contact Robert Brown, MD
Pearl: consider desmopressin (DDAVP) for patients with an intracranial hemorrhage who are taking an antiplatelet. Caution, this is not for patients with an ischemic stroke with hemorrhagic conversion and it was not specifically evaluated for patients on anticoagulation or going to the OR with neurosurgery.
How strong is this evidence? International guidelines already give cautious approval for this practice, and now there is a retrospective review to support it. Though there were only 124 patients in the trial, the rate of hemorrhage expansion was much lower in the DDAVP group (10.9% vs 36.2%, P = .002) and there was no increased risk of hyponatremia (no events reported).
Background: the USPSTF updated recommendations for aspirin for primary prevention of stroke, heart attack, and colon cancer to cut down on over prescription (it's no longer indicated if you're over 70 and it's a question of shared decision-making if you're over 60) but a staggering number of our patients will be on at least one anti-platelet drug when they present with an intracranial hemorrhage.
Past guidelines for treating the bleed in a patient on an anti-platelet drug have given guarded support to giving desmopressin (DDAVP), but some worried the potential for hyponatremia and worsening cerebral edema might outweigh the benefit of releasing von Willebrand Factor.
This study from Upstate University Hospital, Syracuse reviewed 124 cases of intracranial hemorrhage in patients on antiplatelets, but not on anticoagulation and not going to the OR. A total of 55 got DDAVP and 69 did not. The rate of hemorrhage expansion in the first 24 hours was much lower in the DDAVP group (10.9%) than the untreated group (36.2%), and without a significant difference in the rates of hyponatremiia (no events) or thrombotic events (though this last one trended toward more events in the DDAVP group at 7.3% compared to 1.4% in the untreated group).
Feldman E, Meola G, Zyck S, et al. Retrospective Assessment of Desmopressin Effectiveness and Safety in Patients With Antiplatelet-Associated Intracranial Hemorrhage. Critical Care Medicine 2019; 47(12):1759-1765.
Category: Critical Care
Posted: 12/3/2019 by Mike Winters, MBA, MD
(Updated: 2/9/2026)
Click here to contact Mike Winters, MBA, MD
Interventions Shown to Reduce Mortality in RCTs
Santacruz CA, et al. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit Care Med. 2019; 47:1680-1691.
Category: Critical Care
Keywords: conservative oxygenation (PubMed Search)
Posted: 11/26/2019 by Quincy Tran, MD, PhD
(Updated: 2/9/2026)
Click here to contact Quincy Tran, MD, PhD
Settings
Study Results:
Discussion:
This study’s results differed from previous single center study (Girardis JAMA 2016) or meta analysis (Chu DK, Lancer 2018), which showed mortality benefit in patients with conservative oxygen (Girardis & Chu) and more ventilator-free days (Girardis).
Conclusion: Conservative oxygen did not significantly affect the ventilator free days of mechanically ventilated patients.
Reference:
1. ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R, Young P.
Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297. [Epub ahead of print]
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis.
Lancet 2018; 391: 1693-705.
3. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU. randomized clinical trial.
JAMA 2016; 316: 1583-9.
Category: Critical Care
Keywords: OHCA, cardiac arrest, resuscitation, PEA, pesudo-PEA, pulseless electrical activity (PubMed Search)
Posted: 11/12/2019 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
When managing cardiac arrest, it is important to differentiate PEA, the presence of organized electrical activity without a pulse, from "pseudo-PEA,"where there is no pulse but there IS cardiac activity visualized on ultrasound.
Why:
How:
What:
Bottom Line: Pseudo-PEA is different from PEA. Utilize POCUS during your cardiac arrests to identify it and to help diagnose reversible causes, and treat it as a profound shock state with the appropriate supportive measures, i.e. pressors or inotropy.
Rabjohns J, Quan T, Boniface K, Pourmand A. Pseudo-pulseless electrical activity in the emergency department, an evidence based approach. Am J Emerg Med. 2019. DOI:https://doi.org/10.1016/j.ajem.2019.158503
Category: Critical Care
Keywords: Pseudo-PEA, Shock, Resuscitation (PubMed Search)
Posted: 10/29/2019 by Mark Sutherland, MD
Click here to contact Mark Sutherland, MD
Ever been in an acute rescucitation and found yourself unable to remember all of those famous ACLS Hs and Ts? I know I have. A few years ago Littman et al published an alternative approach to critically ill, hypotensive medical patients with non shockable rhythms. Unfortunately, it seems like some of the enthusiasm for this approach has died down, but I still think it's something you're more likely to recall in a pinch than the Hs and Ts and is a better way of getting started with a hypotensive non-trauma patient. And it's so simple you may actually remember it!
1) Look at the monitor. Is the rhythm narrow or wide?
2a) Narrow - more likely a mechanical problem (tamponade, tension PTX, autoPEEP, or PE). Give IVF and search for one of these causes (and correct it!). Keep in mind that ultrasound can help you differentiate a lot of these.
2b) Wide - more likely a metabolic problem (hyperK, sodium channel blockade, etc*). Give empiric calcium, bicarb, and other therapies targeted for these problems (if desired) and get stat labs.
Take a minute and either go to this REBEL EM post:
https://rebelem.com/a-new-pulseless-electrical-activity-algorithm/
To review this, or look at the attached diagrams.
*Dr. Mattu would want me to remind you that hyperkalemia IS a sodium channel poisoned state, so there's no need to think of these two separately
Rebel EM: https://rebelem.com/a-new-pulseless-electrical-activity-algorithm/
Littmann et al. A Simplified And Structured Teaching Tool for the Evaluation and Management of Pulseless Electrical Activity. Med Princ Pract 2014; 23: 1 – 6. PMID: 23949188
Category: Critical Care
Posted: 10/15/2019 by Mike Winters, MBA, MD
(Updated: 2/9/2026)
Click here to contact Mike Winters, MBA, MD
The Critically Ill Geriatric Patient with Sepsis
Khoujah D, et al. Resuscitating the critically ill geriatric emergency department patient. Emerg Med Clin N Am. 2019; 569-81.
Category: Critical Care
Keywords: cardiac arrest, hypothermia, nonshockable rhythm (PubMed Search)
Posted: 10/8/2019 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Rationale: Data regarding temperature management in patients suffered from cardiac arrest with nonshockable rhythm was inconclusive.
Objective: whether moderate hypothermia at 33C, compared with normothermia at 37C would improve neurologic outcome in patients with coma after cardiac arrest with nonshockable rhythm.
Outcome: survival with favorable 90-day neurologic outcome (Cerebral Performance Category scale 1-2/5)
SummaryThere was higher percentage of patients achieving CPC 1-2 in the hypothermia group (10.2%) vs normothermia group (5.7%, Hazard Ratio 4.5, 95% CI 0.1-8.9, p=0.04)
This randomized multicenter trial involved 581 patients with cardiac arrest and nonshockable rhythm. Hypothermia group included 284 patients vs. 297 in the normothermia group. Median GCS at enrollment = 3.
Majority of patients was cooled with the use of a basic external cooling device: 37% for hypothermia and 50.8% for normothermia group.
There was higher percentage of patients achieving CPC 1-2 in the hypothermia group (10.2%) vs normothermia group (5.7%, Hazard Ratio 4.5, 95% CI 0.1-8.9, p=0.04)
Limitation:
A. The study used strict enrollment criteria:
B. normothermia group had higher proportion of patients with temperature at 38C.
C. Hypothermia group underwent temperature management of 56 hours vs. 48 hours for normothermia patients.
Take home points:
In a selected group of patients with cardiac arrest and nonshockable rhythm, moderate hypothermia at 33C may improve neurologic outcome.
Lascarrou JB, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, Coupez E, Dequin PF, Cariou A, Boulain T, Brule N, Frat JP, Asfar P, Pichon N, Landais M, Plantefeve G, Quenot JP, Chakarian JC, Sirodot M, Legriel S, Letheulle J, Thevenin D, Desachy A, Delahaye A, Botoc V, Vimeux S, Martino F, Giraudeau B, Reignier J; CRICS-TRIGGERSEP Group.
Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm.
N Engl J Med. 2019 Oct 2. doi: 10.1056/NEJMoa1906661. [Epub ahead of print]
Category: Critical Care
Posted: 10/1/2019 by Caleb Chan, MD
(Updated: 2/9/2026)
Click here to contact Caleb Chan, MD
Blood Transfusion Thresholds in Specific Populations
Sepsis - 7 g/dL
Acute Coronary Syndrome - no current specific recommendations pending further studies
Stable Cardiovascular Disease - 8 g/dL
Gastrointestinal Bleeds
Acute Neurologic Injury - Traumatic Brain Injury - 7 g/dL
Postpartum Hemorrhage - 1:1:1 ratio strategy
Cable CA, Razavi SA, Roback JD, Murphy DJ. RBC Transfusion Strategies in the ICU: A Concise Review. Crit Care Med. 2019; epub ahead of print.
Category: Critical Care
Keywords: VAPI, acute respiratory failure, vaping, e-cigarettes, e-hookah, juul, pulmonary disease, acute lung diease, ARDS (PubMed Search)
Posted: 9/23/2019 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
The U.S. is currently experiencing an epidemic of a severe lung disease termed Vaping-Associated Pulmonary Illness (VAPI), with over 500 cases and 7 deaths across 38 states and 1 U.S. territory since July 2019.
The clinical presentation of VAPI varies --
Diagnostics --
Treatment is supportive +/- steroids --
Bottom Line: Include vaping-associated pulmonary illness in your differential for patients presenting with acute lung disease.
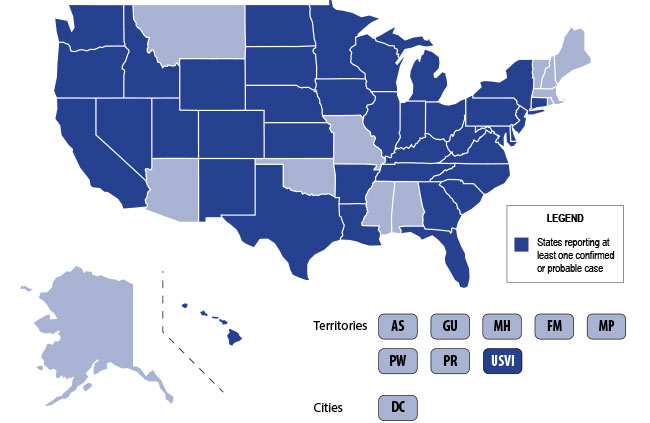
Background: The use of electronic nicotine delivery systems, also known as e-cigarettes or vape pens, has risen precipitously since their introduction in 2006. They heat a liquid that can contain nicotine, THC/CBD, flavors and/or other additives, producing an aersol that is inhaled by users.
They have been marketed as a way to quit smoking, and as being safer than cigarettes. The U.S. is, however, currently experiencing an epidemic of a severe lung disesae termed Vaping-Associated Pulmonary Illness, with over 500 cases and 7 deaths across 38 states and 1 U.S. territory.
Per data obtained by the CDC:
CDC Emergency Preparedness & Response: "Severe Pulmonary Disease Associated with Using E-Cigarette Products" https://emergency.cdc.gov/han/han00421.asp
Category: Critical Care
Keywords: Pregnant, difficult airway (PubMed Search)
Posted: 9/17/2019 by Kim Boswell, MD
Click here to contact Kim Boswell, MD
Most non-OB physicians experience some fear or anxiety over taking care of the average pregnant patient. There are two patients to consider when caring for these women. Critical illness adds another layer of complexity to an already challenging patient population. Due to the normal physiologic changes that occur during pregnancy there are specific and important factors to be aware of when considering and preparing for intubation.
Djabatey EA, Barclay PM. Difficult and failed intubationin 3430 obstetric general anesthesics. Anaesthesia 2009;64: 1168.
Izci B, Vennelle M, Liston WA, et al. Sleep-disordered breathing and upper airway size in pregnancy and post part. Our Respir J 2006; 27:321.
Lebowitz PW, Shay H, Straker T, et al. Shoulder and head elevation improves laryngoscope view for tracheal intubation in non obese as well as obese individuals. J Clin Anesth 2012; 24:104.
Category: Critical Care
Keywords: VAD, LVAD, Heart Failure (PubMed Search)
Posted: 9/9/2019 by Mark Sutherland, MD
(Updated: 9/10/2019)
Click here to contact Mark Sutherland, MD
It's important to remember the differential for the patient with Ventricular Assist Device (VAD) difficulties, as these patients are likely to show up in your ED.
1) Assess the patient as you usually would (signs of life, mental status, breathing, arrhythmias on monitor, etc). Listen for a hum over the chest. Don't expect to feel a pulse.
2) Look at the VAD including controller, driveline, and power source for alarms, disconnections, signs of infection, and other obvious issues.
3) Look at the power (displayed flow), pulsatility index, and pump speed on the controller to help determine the cause of the issue (see attached chart). Once you have a suspected etiology, typical management of these issues is usually similar to non-VAD patients (i.e. gentle IVF for hypovolemia, vasodilators if low flow is due to afterload/hypertension, defibrillation/CPR for arresting pts, etc).
Don't forget to call your VAD coordinator when able. Consider a-line placement for precise evaluation of blood pressure (focus on MAP).
Bottom Line: Consider obstruction/thrombosis, bleeding, infection, hypovolemia, afterload/hypertension, arrhythmia, worsening LV function, and suction events when troubleshooting VADs. The power, pulsatility index, and pump speed help differentiate these conditions.
http://maryland.ccproject.com/2013/12/12/introduction-ventricular-assist-devices/
Category: Critical Care
Keywords: Atrial Fibrillation, sepsis, critical care, cardioversion, beta blockers, calcium channel blockers, rate control, rhythm control (PubMed Search)
Posted: 9/3/2019 by Robert Brown, MD
(Updated: 2/9/2026)
Click here to contact Robert Brown, MD
One third of your critically ill patients will have atrial fibrillation.
More than one third of those patients will develop immediate hypotension because of it.
More than one in ten will develop ischemia or heart failure because of it.
This is what you should know for your next shift:
#1 Don't wait to use electricity. If your patient is hypotensive or ischemic because of atrial fibrillation, you do not need to wait for anticoagulation before you cardiovert.
#2 Electricity buys you time to load meds. Fewer than half of patients you cardiovert will be in sinus rhythm an hour later and fewer than a quarter at the end of a day.
#3 There is no perfect rate control agent. Beta blockers have a lower mortality in A-fib from sepsis. Esmolol has the benefit of being short-acting if you cause hypotension. Diltiazem has better sustained control than amiodarone or digoxin.
#4 There is no perfect rhythm control agent. Magnesium is first-line in guidelines. Amiodarone can be used even when there is coronary artery or structural heart disease.
#5 Anticoagulation is controversial. In sepsis, anticoagulation does not reduce the rate of in-hospital stroke, but does increase the risk of bleeding. Use with caution if cardioversion isn't planned.
Bosch N, Cimini J, Walkey A. Atrial Fibrillation in the ICU. CHEST 2018; 154(6):1424-1434
Category: Critical Care
Posted: 8/27/2019 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Critical Care Management of AIS
Smith M, Reddy U, Robba C, et al. Acute ischaemic stroke: challenges for the intensivist. Intensive Care Med. 2019; epub ahead of print.
Category: Critical Care
Keywords: Torsades de pointes, QT prolongation, antibiotics (PubMed Search)
Posted: 8/20/2019 by Quincy Tran, MD, PhD
(Updated: 2/9/2026)
Click here to contact Quincy Tran, MD, PhD
A new study confirmed the previously-known antibiotics to be associated with Torsades de pointes and QT prolongation (Macrolides, Linezolid, Imipenem and Fluoroquinolones). However, this study found new association between amikacin and Torsades de pointes/QT prolongation.
Methods
The authors queried the United States FDA Adverse Event Reporting System (FAERS) from 01/01/2015 to 12/31/2017 for reports of Torsade de points/QT prolongation (TdP/QT).
Reporting Odd Ratio (ROR) was calculated as the ratio of the odds of reporting TdP/QTP versus all other ADRs for a given drug, compared with these reporting odds for all other drugs present in FAERS
Results
FAERS contained 2,042,801 reports from January 1, 2015 to December 31, 2017. There were 3,960 TdP/QTP reports from the study period (0.19%).
Macrolides ROR 14 (95% CI 11.8-17.38)
Linezolid ROR 12 (95% CI 8.5-18)
Amikacin ROR 11.8 (5.57-24.97)
Imipenem-cilastatin ROR 6.6 (3.13-13.9)
Fluoroquinolones ROR 5.68 (95% CI 4.78-6.76)
Limitations:
These adverse events are voluntary reports
There might be other confounded by concomitant drugs such as ondansetron, azole anti-fungals, antipsychotics.
Teng C, Walter EA, Gaspar DKS, Obodozie-Ofoegbu OO, Frei CR. Torsades de pointes and QT prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. Int J Med Sci. 2019 Jun 10;16(7):1018-1022.
Category: Critical Care
Posted: 8/14/2019 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
The Kidney Transplant Patient in Your ED
Darmon M, Canet E, Ostermann M. Ten tips to manage renal transplant recipients. Intensive Care Med. 2019;45(3):380-383.
Category: Critical Care
Keywords: mechanical ventilation, respiratory failure, obstructive lung disease, asthma exacerbation, COPD (PubMed Search)
Posted: 8/6/2019 by Kami Windsor, MD
Click here to contact Kami Windsor, MD
Managing the intubated patient with exacerbation of severe obstructive lung disease, especially asthma, can be very challenging as it carries higher risks of barotrauma due to higher pulmonary pressures and circulatory collapse due to auto-PEEP and decreased venous return. When measures such as medical therapy and noninvasive positive-pressure ventilation fail to prevent intubation, here are some tips to help:
1. Utilize a volume control ventilation mode to ensure a set tidal volume delivery / minute ventilation, as pressure-targeted modes will be more difficult due to the high pulmonary pressures in acute obstructive lung disease.
2. Set a low RR in order to allow for full exhalation, avoiding air-trapping / breath-stacking and circulatory collapse due to decreased venous return. This may require deep sedation and potentially paralysis.
3. Increase your inspiratory flow by shortening your inspiratory time (thereby increasing your time for exhalation.
4. Monitor for auto-PEEP:
5. Peak inspiratory pressures will be high -- what is more important is the plateau pressure, measured by performing an inspiratory hold at the end of inspiration. Provided your plateau pressure remains <30, you don't need to worry as much about the peak pressure alarms.
6. If your patient acutely decompensates in terms of hemodynamics and oxygenation -- first attempt to decompress their likely auto-PEEPed lungs by popping them off the ventilator and manually press on their chest to assist with exhalation of stacked breaths allowing venous return to the heart.
Category: Critical Care
Keywords: Mechanical Ventilation, Intubation, Extubation, RSBI (PubMed Search)
Posted: 7/28/2019 by Mark Sutherland, MD
(Updated: 7/30/2019)
Click here to contact Mark Sutherland, MD
With increasing critical care boarding and the opioid crisis leading to more intubations for overdose, extubation - which was once a very rare event in the ED - is taking place downstairs more often. Prolonged mechanical ventilation is associated with a ton of complications, so it's important for the ED physician to be comfortable assessing extubation readiness. There is no single accepted set of criteria, but most commonly used are some variant of the following:
If the above criteria are met, two additional tests are frequently considered:
And don't forget to consider extubating high risk patients directly to BiPAP or HFNC!
Bottom Line: For conditions requiring intubation where significant clinical improvement may be expected while in the ED (e.g. overdose, flash pulmonary edema, etc), be vigilant about, and have a system for, assessing readiness for extubation.
1. Souter MJ, Manno EM. Ventilatory management and extubation criteria of the neurological/neurosurgical patient. The Neurohospitalist. 2013;3(1):39-45. doi:10.1177/1941874412463944
2. Thille AW, Richard J-CM, Brochard L. Concise Clinical Review The Decision to Extubate in the Intensive Care Unit. doi:10.1164/rccm.201208-1523CI
3. Ouellette DR, Patel S, Girard TD, et al. Liberation From Mechanical Ventilation in Critically Ill Adults: An Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: Inspiratory Pressure Augmentation During Spontaneous Breathing Trials, Protocols Minimizing Sedation, and Noninvasive Ventilation Immediately After Extubation. Chest. 2017;151(1):166-180. doi:10.1016/j.chest.2016.10.036
Category: Critical Care
Keywords: empyema (PubMed Search)
Posted: 7/23/2019 by Robert Brown, MD
Click here to contact Robert Brown, MD
The incidence of empyema as a complication of pneumonia has been increasing since the 1990's and source control requires removing the pus from the chest as soon as possible, but how large should the drain be? The American Association for Thoracic Surgery (AATS) released the most recent guidelines for identifying and managing empyema in June 2017 and at the time had no certain evidence to guide the choice of large-bore vs small-bore catheters. Most studies to guide us are flawed (not randomized), but no recently published randomized studies exist to provide a definitive answer.
Bottom line: a small-bore pigtail catheter is a reasonable choice to drain empyema and flushing it every 6 hours has been shown to prevent clogging.
Category: Critical Care
Posted: 7/16/2019 by Mike Winters, MBA, MD
(Updated: 2/9/2026)
Click here to contact Mike Winters, MBA, MD
POCUS in the Critically Ill Pregnant Patient
Blanco P, Abdo-Cuza A. Point-of-care ultrasound in the critically ill pregnant or postpartum patient: what every intensivist should know. Intensive Care Med. 2019; epub ahead of print.
Category: Critical Care
Keywords: Critical Care, Hypotension, Shock, Vasopressors (PubMed Search)
Posted: 7/9/2019 by Mark Sutherland, MD
(Updated: 2/9/2026)
Click here to contact Mark Sutherland, MD
With a shortage of push dose epi, this may be an opportune time to review alternative options (see also Ashley's email on the subject).
The dose of vasopressor required to reverse hypotension has been most studied in pregnant women undergoing c-section who get epidurals and experience spinal-induced vasoplegia and hypotension (not necessarily our patient population, but we can extrapolate...)
Phenylephrine was found to reverse hypotension 95% of the time at a dose of 159 micrograms (a neo stick has 100 ug/mL, so around 1-2 mL out of the stick)
Norepinephrine reversed hypotension in 95% of patients at a dose of 5.8 ug. The starting dose for our norepi order in Epic is 0.01 ug/kg/min, so if you have a levophed drip hanging and have an acutely hypotensive patient, you may want to briefly infuse at a higher rate such as 0.1 ug/kg/min (for a typical weight patient), or bolus approximately 3-7 ug for a typical patient. Of course the degree of hypotension, particular characteristics of your patient and clinical context should be taken into consideration. When your a lucky enough to have this resource, always consult your pharmacist.
Bottom Line: To reverse acute transient hypotension you may consider:
-A bolus of phenylephrine 50-200 ug (0.5-2 mL from neo-stick)
-A bolus of norepinephrine 3-7 ug
-Briefly increasing your norepinephrine drip (if you have one) to something around 0.1 ug/kg/min in a typical weight patient
-Always search for other causes of hypotension and consider clinical context.
Onwochei DN, Ngan kee WD, Fung L, Downey K, Ye XY, Carvalho JCA. Norepinephrine Intermittent Intravenous Boluses to Prevent Hypotension During Spinal Anesthesia for Cesarean Delivery: A Sequential Allocation Dose-Finding Study. Anesth Analg. 2017;125(1):212-218. (https://www.ncbi.nlm.nih.gov/pubmed/28248702)
Tanaka M, Balki M, Parkes RK, Carvalho JC. ED95 of phenylephrine to prevent spinal-induced hypotension and/or nausea at elective cesarean delivery. Int J Obstet Anesth. 2009;18(2):125-30. (https://www.ncbi.nlm.nih.gov/pubmed/19162468)
Weingart S. Push-dose pressors for immediate blood pressure control. Clin Exp Emerg Med. 2015;2(2):131–132. Published 2015 Jun 30. doi:10.15441/ceem.15.010 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052865/)
